Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by eczematous lesions and often intense pruritus and atopic and non-atopic comorbidities [1, 2]. Its prevalence of 3% to 10% in adults and up to 20% in children makes it the most common chronic skin condition [3, 4]. The burden of AD on patients includes substantial psychosocial distress and systemic comorbidities [4, 5]. Various factors contribute to the occurrence of AD, such as skin barrier defects, activation of the Th2 immune pathway [6], and bacterial diversity [7].
As a chronic disease, moderate-to-severe AD often requires long-term treatment. However, data on efficacy and safety of long-term treatment are scare [8–10]. Three systemic therapeutic options have been approved for the treatment of patients with severe disease who are not controlled by topic medications, including oral corticosteroids, oral cyclosporine, and UVA/narrow-band UVB phototherapy [11]. However, neither of these treatments is suitable for long-term treatment due to their limited efficacy and unfavourable safety [12–15]. Therefore, it is necessary to find new treatment regimens that could provide effective and safe long-term therapies for moderate-to-severe AD [14, 16].
Dupilumab is a fully human monoclonal antibody that blocks the shared receptor unit for interleukin-4 and interleukin-13, inhibiting the signalling of interleukin 4 and interleukin 13, type 2/Th2 inflammatory cytokines [17]. Dupilumab has been approved by the US Food and Drug Administration (FDA) for use in patients with moderate-to-severe AD. Although several clinical trials have confirmed the efficacy of dupilumab for moderate-to-severe AD, their convinced evidence is not sufficient to draw a robust conclusion because of the limited sample size, inconsistent results, as well as different dosage administered.
Aim
We conducted this meta-analysis to evaluate the efficacy and safety of dupilumab in patients with moderate-to-severe AD.
Material and methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and meta-analysis (PRISMA) criteria [18].
Search strategy
To identify all available studies, a detailed search of studies reporting the efficacy and safety of dupilumab treatment in AD was performed by two authors. A systematic search was conducted in major electronic databases (PubMed, Web of Science, and Embase), using the following search terms: “dupilumab” and “atopic dermatitis” or “atopic eczema”. These databases were searched for articles published between 1975 and March 2021. The search was performed without publication status and language restrictions. In order to retrieve addition references, we used Citation Pearl Growing strategy [19]. An addition manual search was performed within the references provided in included manuscripts until no potential studies were found. The corresponding author would be contacted by email when original data could not be retrieved from the study.
Study inclusion
The inclusion criteria were developed using the PICOS framework [18]. The studies were included in this meta-analysis if they met the following inclusion criteria: (1) patients diagnosed with moderate-to-severe AD; (2) intervention treatment must include dupilumab; (3) control treatment could be placebo or any other treatment; (4) reporting at least one of the following outcomes: Investigator’s Global Assessment response (IGA), Eczema Area and Severity Index (EASI), the pruritus numeric rating scale (NRS), percent BSA affected with AD, Dermatology Life Quality Index (DLQI) and adverse events; (5) studies were performed as a randomized controlled trial (RCT). When publications from the same trial appeared, only the one featured the longest duration of follow-up, or the most recent study, was included.
Data extraction and risk of bias assessment
A specifically designed data extraction sheet was used to extract data from original trials. The following details were extracted whenever available: study characteristics (first author, country, year of publication, study design, sample size, duration of follow-up), patient characteristics (inclusion criteria, mean age, dosage of dupilumab, and outcome measures). To minimize data entry error, all data were entered in duplicate by two individuals separately, and were cross-checked to ensure the accuracy of data. Any disparity would be discussed and resolved by a group meeting.
To evaluate the risk of bias in the included studies, a risk of bias assessment tool was applied [20]. Five domains were used to assess the quality of each RCT including the random sequence generation; allocation concealment; blinding of outcome participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting and other bias [20]. Each domain was graded, and the risk of bias was scored as a low risk of bias, unclear risk of bias and high risk of bias following the descriptors of the tool [20].
Statistical analysis
All statistical analyses were carried out using STATA version 12.0 (Stata Corporation, College Station, TX, USA). Differences in continuous variables were expressed as standard mean difference (WMD) with 95% confidence intervals (CIs); differences in dichotomous variables were expressed as risk ratio (RR) with 95% CIs. Statistical heterogeneity was assessed using Cochrane Q and I 2 statistic, in which p < 0.1 or I 2 > 50% were considered to be significant [21]. A fixed-effects model [22] or random-effects model [23] was employed to pool the estimate according to the heterogeneity among the included studies. When substantial heterogeneity was identified, sensitivity analysis was conducted to evaluate the stability of synthesis results and explore the potential sources of heterogeneity. We also performed subgroup analysis of results according to the interval of dupilumab dose, treatment regimen and treatment duration. Publication bias was evaluated using Begg’s [24] and Egg’s [25] tests. A p-value less than 0.05 was judged as statistically significant except where a certain p-value had been given.
Meta-regression analysis
Due to the limited data, we only performed meta-regression analysis for IGA response. We hypothesized that differences among included studies might be influenced by the demographic (age and gender), clinical (IGA score and EASI score at baseline, treatment duration, time interval of dupilumab between two doses, treatment regimen) variables. In order to explain whether these variables have a possible effect on IGA response, we performed meta-regression analysis. In this regression model, IGA response was chosen as a dependent variable (y) and the variables mentioned above were chosen as independent variables (χ). This analysis was performed with a random-effects model.
Results
Study selection
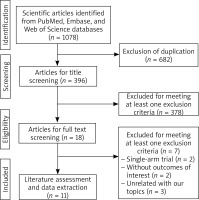
The initial search identified 1078 papers. After duplicate exclusion, 396 underwent title/abstract review, leaving 18 studies screened for eligibility. Of these, seven studies were excluded for the following reasons: three articles were comparative studies rather than RCTs, two studies reported data out of our interest (cost burden analysis), and two were single-arm studies. Finally 11 studies [26–36] met the criteria for inclusion (Figure 1). No additional papers were retrieved from previous studies.
Study characteristics and risk of bias
Table 1 presented the detailed characteristics of included studies. These studies were published between 2014 and 2021. The sample size in the individual studies ranged from 64 to 740 participants, with a total of 4094 participants. The duration of treatment ranged from 12 to 76 weeks. Dupilumab was administered with 300 mg qw in eight studies [26–31, 33, 35], 300 mg q2w in four studies [27, 28, 30, 35, 36], 300 mg q4w in three studies [26, 32, 35], and 300 mg q8w in one study [32]. Dupilumab was used as monotherapy in eight studies [26, 27, 29, 31–34, 36] and as a combination therapy in three studies [28, 30, 35].
Table 1
Baseline characteristics of patients in the trials included in the meta-analysis
| Study | Country | Treatment regimen | Dosing | No. of patients | Treatment duration |
|---|---|---|---|---|---|
| Paller AS [26] | China | Dupilumab | 300 mg q4w, 200/300 mg q2w | 84 | 16 weeks |
| Placebo | 85 | ||||
| Simpson EL [27] | USA | Dupilumab | 300 mg qw, 300 mg q2w | 447 | 16 weeks |
| Placebo | 224 | ||||
| Blauvelt A [28] | USA | Dupilumab + TCS | 300 mg qw, 300 mg q2w | 425 | 52 weeks |
| Placebo + TCS | 315 | ||||
| Blauvelt A [29] | USA | Dupilumab | 300 mg qw | 97 | 16 weeks |
| Placebo | 97 | ||||
| de Bruin-Weller M [30] | Netherlands | Dupilumab + TCS | 300 mg qw, 300 mg q2w | 217 | 16 weeks |
| Placebo + TCS | 108 | ||||
| Deleuran M [31] | Denmark | Dupilumab | 300 mg qw | 249 | 76 weeks |
| Placebo | 398 | ||||
| Worm M [32] | Germany | Dupilumab | 300 mg qw/q2w, 300 mg q4w, 300 mg q8w | 339 | 36 weeks |
| Placebo | 83 | ||||
| Tsianakas A [33] | Germany | Dupilumab | 300 mg qw | 32 | 12 weeks |
| Placebo | 32 | ||||
| Beck LA [34] | USA | Dupilumab | 300 mg qw | 55 | 16 weeks |
| Placebo | 54 | ||||
| Thaci D [35] | Germany | Dupilumab + TCS | 300 mg qw, 300 mg, q2w, 300 mg q4w, 200 mg qw, 200 mg q2w, 100 mg q4w | 318 | 16 weeks |
| Placebo + TCS | 61 | ||||
| Bieber T | Germany | Dupilumab | 200 mg q2w | 243 | 12 weeks |
| Placebo | 131 |
The assessment of risk of bias is shown in Figure 2. All the included studies were performed with a randomized, double-blinded, placebo/placebo+ TCS- controlled design. The methods of random sequence generation and allocation concealment, and the outcome data were all adequately reported. Therefore, all the included studies were regarded as being at low risk of bias. It should be noted that all the trials were funded by pharmaceutical companies. Thus, caution should be taken when interpreting our results.
IGA response (IGA 0/1)
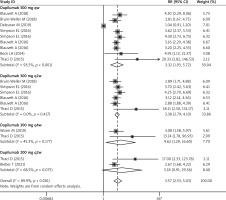
All the included studies reported the data of IGA response [26–36]. Pooled data showed that significantly more patients receiving dupilumab than those receiving other treatments achieved an IGA score of 0 or 1 and an improvement of 2 points or more on the IGA from baseline score (RR = 3.57, 95% CI: 2.53, 5.03; p < 0.001) (Figure 3). The test for heterogeneity was significant, therefore we performed sensitivity (I2 = 89.9%, p < 0.001). When we excluded the trial with a small sample size [34], the summarized data did not have a relatively large change (RR = 4.12, 95% CI: 3.76, 6.18; p < 0.001), but significant heterogeneity remained (I2 = 82.3%, p < 0.001). When the trial with an outlier was removed, the pooled RR altered slightly (RR = 3.82, 95% CI: 3.24, 5.66; p < 0.001), but heterogeneity was still present (I2 = 85.7%, p < 0.001). Moreover, obvious heterogeneity still existed after we removed any single study at each time (data not shown).
Subgroup analysis based on the time interval for dupilumab showed that a higher proportion of patients achieved IGA response (IGA 0/1) in the dupilumab group than in the control group, no matter what the interval between the two doses was (300 mg qw: RR = 3.32, 95% CI: 1.93, 5.72, p < 0.001; 300 mg q2w: RR = 3.38, 95% CI: 2.79, 4.10, p < 0.001; 300 mg q4w: RR = 4.62, 95% CI: 1.29, 16.60, p < 0.001) (Figure 3).
Subgroup analysis based on the treatment regimen suggested that both dupilumab administered as monotherapy (RR = 3.18, 95% CI: 1.84, 5.50; p < 0.001) and in combination with TCS (RR = 3.26, 95% CI: 2.67, 3.98; p < 0.001) was associated with a greater effect in IGA response (IGA 0/1) as compared with other treatments.
Subgroup analysis based on treatment duration showed that dupilumab resulted in a better IGA response (IGA 0/1) than other treatments at 12 weeks (RR = 2.81, 95% CI: 1.81, 4.37; p < 0.001) and 16 weeks (RR = 3.63, 95% CI: 3.10, 4.26; p < 0.001).
EASI score
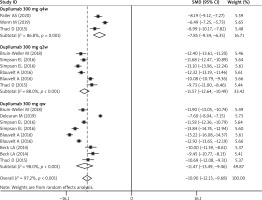
Nine studies reported the data of EASI score [26–28, 30–35]. As shown in Figure 4, the least-squares (LS) mean change in EASI score from baseline was significantly greater among patients receiving dupilumab than among those receiving other treatments (SMD = –10.90, 95% CI: –12.13, –9.68; p < 0.001). There was significant heterogeneity among the included studies (I2 = 97.2%, p < 0.001). We performed sensitivity analysis by excluding the trial with a small sample size [33], the overall estimate changed a little (SMD = –11.52, 95% CI: –13.84, –9.72; p < 0.001), but heterogeneity was still present (I2 = 95.1%, p < 0.001). When we excluded the trial with an outlier, the corresponding pooled data were not materially altered (SMD = –12.73, 95% CI: –14.16, –11.59; p < 0.001), but the heterogeneity did not disappear (I2 = 93.8%, p < 0.001). We then deleted a single study at each time, however, the pooled result and heterogeneity were not altered significantly (data not shown).
Subgroup analysis based on the time interval of two doses showed that dupilumab resulted in a significantly greater reduction in LS mean change in EASI score from baseline as compared with other treatments, regardless of the interval between two doses (300 mg qw: SMD = –11.47, 95% CI: –13.49, –9.46, p < 0.001; 300 mg q2w: SMD = –11.57, 95% CI: –12.64, –10.49, p < 0.001; 300 mg q4w: SMD = –7.85, 95% CI: –9.34, –6.35, p < 0.001) (Figure 4).
Subgroup analysis based on the treatment regimen suggested that both dupilumab administered as monotherapy (SMD = –10.11, 95% CI: –11.56, –8.67; p < 0.001) and in combination with TCS (SMD = –12.47, 95% CI: –13.96, –10.99; p < 0.001) had a significantly greater reduction in EASI score from baseline, compared to other treatments.
Subgroup analysis based on the treatment duration showed that LS mean change in EASI score from baseline was significantly greater in patients receiving dupilumab at 16 weeks (SMD = –10.11, 95% CI: –11.56, –8.67; p < 0.001) and 52 weeks (SMD = –11.50, 95% CI: –14.29, –8.71; p < 0.001), when compared with those receiving other treatments.
Percentage of BSA
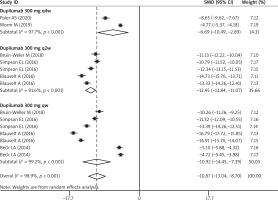
Six studies reported the data of percentage of BSA affected [26–28, 30, 32, 34]. The LS mean percent change in BSA from baseline was significantly greater among patients receiving dupilumab than among those receiving other treatments (SMD = –10.87, 95% CI: –13.04, –8.70; p < 0.001) (Figure 5). The test for heterogeneity was significant (I2 = 98.7%, p < 0.001).
Subgroup analysis based on the time interval for dupilumab showed that patients in the dupilumab group achieved a greater percentage of BSA than those in the control group, no matter what the interval between two doses was (300 mg qw: SMD = –10.92, 95% CI: –14.45, –7.39, p < 0.001; 300 mg q2w: SMD = –12.45, 95% CI: –13.84, –11.07, p < 0.001; 300 mg q4w: SMD = –6.69, 95% CI: –10.49, –2.89, p < 0.001) (Figure 5).
Subgroup analysis based on the treatment regimen suggested that both dupilumab administered as monotherapy (SMD = –8.88, 95% CI: –11.43, –6.33; p < 0.001) and in combination with TCS (SMD = –13.53, 95% CI: –15.46, –11.60; p < 0.001) were associated with a greater effect in percentage of BSA as compared with other treatments.
Pruritus NRS score
Eight studies reported the data of pruritus NRS score [26–31, 34, 35]. The LS mean percent change in pruritus NRS score was significantly greater among patients receiving dupilumab than among those receiving other treatments (SMD = –9.29, 95% CI: –10.34, –8.25; p < 0.001). There was significant heterogeneity among the included studies (I2 = 96.6%, p < 0.001).
Subgroup analysis based on the time interval for dupilumab showed that dupilumab resulted in a significantly greater reduction in percent change in pruritus NRS score as compared with other treatments, regardless of the interval between two doses (300 mg qw: SMD = –9.62, 95% CI: –11.26, –7.99, p < 0.001; 300 mg q2w: SMD = –9.80, 95% CI: –10.78, –8.82, p < 0.001).
Subgroup analysis based on the treatment regimen suggested that both dupilumab administered as monotherapy (SMD = –9.27, 95% CI: –10.69, –7.86; p < 0.001) and in combination with TCS (SMD = –9.31, 95% CI: –10.81, –7.80; p < 0.001) had a significantly greater reduction in percent change in pruritus NRS score, compared to other treatments.
DLQI
Seven studies reported the data of DLQI [26–28, 30–32, 35]. The mean change in DLQI score was significantly greater among patients receiving dupilumab than among those receiving other treatments (SMD = –9.66, 95% CI: –11.50, –7.82; p < 0.001). There was significant heterogeneity among the included studies (I2 = 99.1%, p < 0.001).
Subgroup analysis based on the time interval for dupilumab showed that dupilumab resulted in a significantly greater reduction in the change of DLQI score as compared with other treatments, regardless of the interval between two doses (300 mg qw: SMD = –10.67, 95% CI: –14.23, –7.10, p < 0.001; 300 mg q2w: SMD = –10.34, 95% CI: –12.42, –8.25, p < 0.001; 300 mg q4w: SMD = –5.96, 95% CI: –6.65, –5.26, p < 0.001).
Subgroup analysis based on the treatment regimen suggested that both dupilumab administered as monotherapy (SMD = –8.50, 95% CI: –10.86, –6.13; p < 0.001) and in combination with TCS (SMD = –10.57, 95% CI: –13.09, –8.05; p < 0.001) had a significantly greater reduction in the change of DLQI score, compared to other treatments.
Adverse events
All the included studies reported the data of adverse events [26–36], however, only eight of these studies provided available data [27–30, 32–35]. The incidence of adverse events was comparable between the dupilumab and control groups (RR = 1.00, 95% CI: 0.96, 1.03; p = 0.832). There was no significant heterogeneity across the included studies (I2 = 0.0%, p = 0.491). Subgroup analysis based on the time interval for dupilumab revealed similar results, which suggested no significant differences between the 300 mg qw dose (RR = 0.98, 95% CI: 0.93, 1.03; p = 0.430), 300 mg q2w dose (RR = 1.02, 95% CI: 0.96, 1.08; p = 0.526) and control treatment.
The most common adverse events, including injection-site reaction (RR = 2.45, 95% CI: 1.63, 3.75; p < 0.01), headache (RR = 1.23, 95% CI: 1.07, 2.54; p = 0.039), and conjunctivitis (RR = 3.26, 95% CI: 2.01, 4.77; p < 0.001), were more frequently seen in the dupilumab-treatment group.
Meta-regression analysis
In order to further explore the effect of dupilumab on moderate-to-severe AD, we performed some meta-regression analysis. Results showed that none of these variables affected the difference in IGA response between dupilumab and control treatments (age: Z = 2.31, p = 0.052; gender: Z = 3.25, p = 0.102; IGA score at baseline: Z = –2.15, p = 0.372; EASI score at baseline: Z = –3.18, p = 0.365; treatment duration: Z = –0.33, p = 0.079; time interval of dupilumab between two doses: Z = –1.21, p = 0.315; treatment regimen: Z = 1.38, p = 0.225).
Discussion
This meta-analysis was performed to investigate the efficacy and safety of dupilumab in the treatment of patients with moderate-to-severe AD. Overall, our study suggested that dupilumab was associated with better results than other treatments across all the evaluated measures that reflected the objective signs and subjective symptoms, and the quality of life. Moreover, in the subgroup analysis, dupilumab administered as 300 mg qw or q2w or q4w all showed benefit effects in these outcomes. Dupilumab used as monotherapy or in combination with TCS, was associated with improvements in the skin lesions and pruritus. The adverse events were comparable between dupilumab and other treatments. Our results confirmed the favourable efficacy and acceptable safety of dupilumab in the treatment of patients with moderate-to-severe AD.
There are two similar meta-analyses about the effect and safety assessment of dupilumab in patients with moderate-to-severe AD. Wang et al. [37] carried out a meta-analysis of six RCTs in 2017 and they concluded that dupilumab had an acceptable safety and favourable effects in improving the signs and symptoms of AD. Both 300 mg qw and q2w dupilumab had similar benefits. Another meta-analysis investigated the effect of dupilumab on adverse events in 2018 and showed that dupilumab significantly reduced the risk of skin infection and exacerbation of AD while increased the risk of headache and conjunctivitis [38]. This meta-analysis expands on the previous studies and provides a more comprehensive description of the effects and safety of dupilumab. First, this study had a larger sample size than the previous studies. The last search update of the two previous meta-analyses was in September 2017 and December 2017, and they totally identified six RCTs with 2447 patients and eight RCTs with 2705 patients, respectively. In this study, we additionally included five trials with a total number of 4094 patients, including R668-AD-1526 LIBERTY AD ADOL trial [26], LIBERTY AD CAFÉ trial [30], NCT01949311 trial [31], LIBERTY AD SOLO-CONTINUE trial [32], and the NCT03720470 trial [36]. Our sample size was much larger than the previous analysis, which enhanced the statistical power to assess the effects. Second, we also investigated the effect of dupilumab 300 mg q4w and its long-term efficacy (52 weeks), and the impacts of several variables on IGA response in a meta-regression analysis, which had not been analysed in the previous meta-analyses. In this study, we searched multiple databases with the newest data and largest sample size. Compared with previous reviews, we provided more exact and powerful results on the effect and safety of dupilumab.
Our study demonstrated that subgroup analysis based on the interval between two doses of dupilumab did not affect the results of each clinical outcome measure, in which dupilumab administered with 300 mg qw or 300 mg q2w or 300 mg q4w resulted in the benefit effect on AD. Although these outcomes had small differences between different dose regimens, they still did not reach the minimal clinically important difference.
Our findings confirm and expand on the results of previous meta-analysis of dupilumab in patients with moderate-to-severe AD. IGA, EASI, and BAS affected are objective indices for the evaluation of disease severity and extent; DLQI scores are based on patients’ assessment of quality of life [34]. Therefore, the assessment of response to treatment is based on different perspectives. Our results suggested that marked improvements were observed with the treatment of dupilumab in each of the clinical measures, including decrease in the severity and extent of AD, and improvements in patients’ experience of their symptoms. In addition, the pruritus measured by pruritus NRS, was also improved by dupilumab. Pruritus is considered as a significant contributor to the effect of AD on the quality of life [39, 40], and the improvement on pruritus by dupilumab could be life-changing for these patients.
In the study, we found the overall incidence of adverse events was comparable between dupilumab and other treatment groups. This is consistent with the finding of the previously published trials [27, 29, 30, 33]. Conjunctivitis was more frequently seen in patients receiving dupilumab than those receiving other treatments. The cause of conjunctivitis in AD patients is not fully clear. However, in the early studies in which dupilumab was applied in patients with asthma [41, 42] or chronic sinusitis with nasal polyposis [43], the incidence of conjunctivitis was not increased. This indicated that conjunctivitis might be caused by the specific characteristics of AD rather than dupilumab. There is a need for further studies focusing on the cause of conjunctivitis to address this issue.
There were severe potential limitations. First, substantial heterogeneity was identified in the outcomes across the included studies. In order to explore the potential heterogeneity and the impacts of several variables on the outcomes, we performed sensitivity analysis and meta-regression analysis. However, no meaningful information was found in the data analysis. Second, the evidence for the effect of dupilumab 300 mg q4w might not be sufficient because of the sparse data among the included studies. There is a need for further studies with adequate sample size to confirm these findings. Third, it is worth noting that all the included trials were fully or partially funded by pharmaceutical companies, and their results might be influenced by the commercial interests of the sponsors. Therefore, caution is warranted in the interpretation of our results.
Conclusions
Our findings provided evidence that dupilumab was an effectively targeted biologic therapy in the treatment of patients with moderate-to-severe AD because it ameliorated the signs and symptoms of AD and improved health-related quality of life. Moreover, its safety was acceptable. Considering the potential limitations in this study, more large-scale RCTs are needed to verify our findings.