Introduction
Atopic dermatitis, a chronic and relapsing inflammatory skin condition, results in intense pruritus [1–5]. Its complex pathophysiology involves the interplay of impaired skin barrier function, immune dysregulation, genetic susceptibility, and environmental factors [6–8]. Atopic dermatitis is estimated to affect up to 20% of children and adolescents and up to 10% of adults. These patients have considerable impairment in quality of life, sleep, depression, anxiety, and work absenteeism [9–11].
Management of atopic dermatitis needs systemic therapy, and there is still a lack of effective treatment for some patients with atopic dermatitis [12–14]. Abrocitinib (called PF04965842), an oral Janus kinase (JAK) 1 selective inhibitor, reveals some potential for the treatment of atopic dermatitis. Oral abrocitinib was documented to be effective and well tolerated in a dose-ranging phase 2b study in adults with moderatetosevere atopic dermatitis with regard to the improvement in Investigator Global Assessment (IGA) response and Eczema Area and Severity Index (EASI) score [15].
However, the efficacy of abrocitinib versus placebo for atopic dermatitis has not been well established. Recently, several studies on the topic have been published [16–18].
Aim
With accumulating evidence, we therefore performed a systematic review and meta-analysis of RCTs to explore the efficacy of abrocitinib for patients with atopic dermatitis.
Material and methods
Ethical approval and patient consent are not required because this is a systematic review and meta-analysis of previously published studies. The systematic review and meta-analysis was conducted and reported in adherence to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [19].
Search strategy and study selection
Two investigators independently searched the following databases (inception to June 2021): PubMed, Embase, Web of Science, EBSCO, and Cochrane Library databases. The electronic search strategy was conducted using the following keywords: “abrocitinib” AND “atopic dermatitis”. We also checked the reference lists of the screened full-text studies to identify other potentially eligible trials.
The inclusive selection criteria were as follows: (i) population: patients with atopic dermatitis; (ii) intervention: abrocitinib at the dose of 200 mg once daily; (iii) comparison: placebo; (iv) study design: RCT.
Data extraction and outcome measures
We extracted the following information: author, number of patients, age, female, duration of atopic dermatitis, Eczema Area and Severity Index (EASI) score and detailed methods in each group, etc. Data were extracted independently by two investigators, and discrepancies were resolved by consensus. We also contacted the corresponding author to obtain the data when necessary.
The primary outcomes were IGA response and EASI-75. Secondary outcomes included EASI-90, NRS response, adverse events and serious adverse events.
Quality assessment in individual studies
Methodological quality of the included studies was independently evaluated using the modified Jadad scale [20]. There are 3 items for the Jadad scale: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points). The score of the Jadad scale varies from 0 to 5 points. An article with a Jadad score ≤ 2 is considered to be of low quality. If the Jadad score ≥ 3, the study is considered to be of high quality [21].
Statistical analysis
We estimated the odds ratio (OR) with 95% CIs for all dichotomous outcomes. A random-effects model was used regardless of heterogeneity. Heterogeneity is reported using the I 2 statistic, and I 2 > 50% indicates significant heterogeneity [22]. Whenever significant heterogeneity was present, we searched for potential sources of heterogeneity via omitting one study in turn for the meta-analysis or performing subgroup analysis. All statistical analyses were performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
Results
Literature search, study characteristics and quality assessment
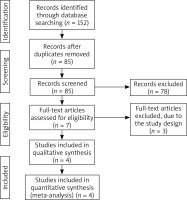
A detailed flowchart of the search and selection results is shown in Figure 1. One hundred and fifty-two potentially relevant articles were identified initially. Finally, four RCTs that met our inclusion criteria were included in the meta-analysis [15–18].
The baseline characteristics of the four eligible RCTs in the meta-analysis are summarized in Table 1. The six studies were published between 2019 and 2021, and total sample size is 932. The intervention treatments are 200 mg of abrocitinib once daily versus placebo for 12 weeks.
Table 1
Characteristics of included studies
Among the four studies included here, four studies report the Investigator’s Global Assessment (IGA) response and EASI-75 [15–18], three studies report the EASI-90 and Numerical Rating Scale (NRS) response [15, 17, 18], three studies report adverse events and serious adverse events [16–18]. Jadad scores of the four included studies vary from 4 to 5, and all four studies are considered to be high-quality ones according to quality assessment.
Primary outcomes: IGA response and EASI-75
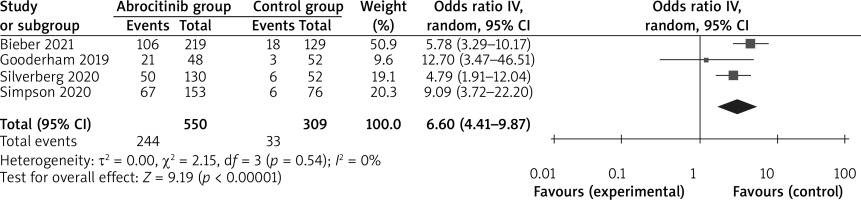
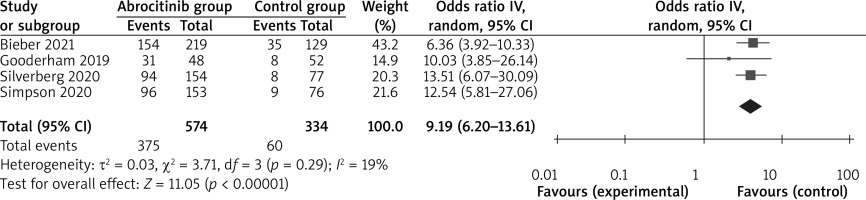
These outcome data were analyzed with the random-effects model, and compared to the control group, for atopic dermatitis, abrocitinib results in a significantly higher IGA response (OR = 6.60; 95% CI: 4.41–9.87; p < 0.00001) with no heterogeneity among the studies (I 2 = 0%, heterogeneity p = 0.54) (Figure 2) and EASI-75 (OR = 9.19; 95% CI: 6.20–13.61; p < 0.00001) with low heterogeneity among the studies (I2 = 19%, heterogeneity p =0.29) (Figure 3).
Sensitivity analysis
No significant heterogeneity was observed among the included studies, and thus we did not perform sensitivity analysis via omitting one study in turn.
Secondary outcomes
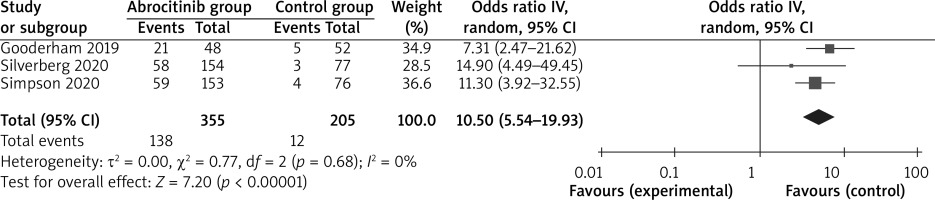
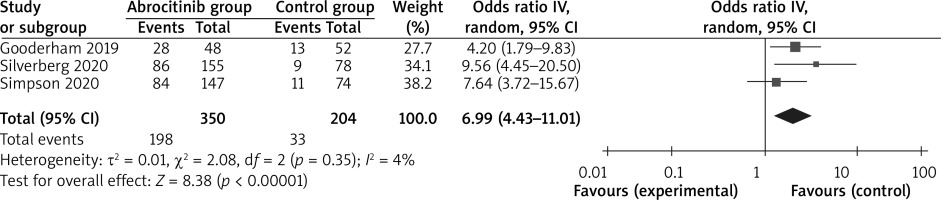
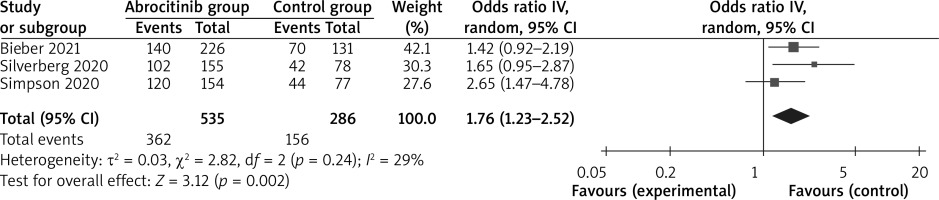
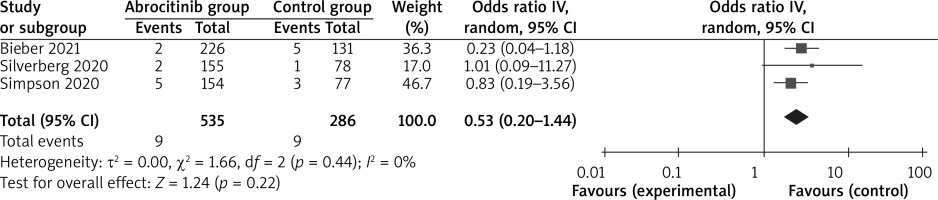
In comparison with the control group for atopic dermatitis, abrocitinib is associated with substantially improved EASI-90 (OR = 10.50; 95% CI: 5.54–19.93; p < 0.0001; Figure 4), NRS response (OR = 6.99; 95% CI: 4.43–11.01; p < 0.00001; Figure 5) and adverse events (OR = 1.76; 95% CI: 1.23–2.52; p = 0.002; Figure 6), but no obvious impact on serious adverse events was revealed (OR = 0.53; 95% CI: 0.20–1.44; p = 0.22; Figure 7).
Discussion
Systemic corticosteroids may have higher efficacy than topical treatments in patients with moderate to severe atopic dermatitis, but is limited by short-term and long-term side effects, and long-term use is not recommended [23]. Other treatment options include immunosuppressive drugs (e.g. ciclosporin, methotrexate, azathioprine, and mycophenolate mofetil), but they are not approved due to adverse events and poor tolerability [23]. One phase 2b trial and two phase 3 placebo-controlled trials demonstrated that 12 weeks of monotherapy with abrocitinib resulted in better outcomes for atopic dermatitis than placebo [15, 17, 18].
Our meta-analysis included four RCTs and 932 patients with atopic dermatitis. The results showed that abrocitinib at the dose of 200 mg once daily promoted a significant improvement in IGA response, EASI-75, EASI-90 and NRS response compared to placebo. Abrocitinib, a small-molecule JAK1 inhibitor, can be administered orally once daily, and promotes the treatment efficacy through inhibiting signaling of interleukin-4, interleukin-13, and other cytokines involved in the pathogenesis of atopic dermatitis [24]. Abrocitinib was reported to be less likely to stimulate an immunogenic response than biologic treatment [25, 26].
Considering the adverse events of abrocitinib at the dose of 200 mg once daily, our results showed obviously more total adverse events than placebo, but these adverse events were generally mild and acceptable. These increased adverse events mainly include nausea and headache [17]. However, the incidence of serious adverse events was not increased by abrocitinib treatment. JAK inhibition can potentially increase the risk of infections due to the involvement of JAKs in signaling pathways that regulate host defense and the immune response [27]. However, one RCT revealed that the incidence of serious infections and herpes virus infections was low. No cases of malignancy were seen [17].
This meta-analysis has several potential limitations. Firstly, our analysis is based on only four RCTs, and more RCTs with a large sample size should be conducted to explore this issue. Secondly, considering the sensitivity analysis, although there is no significant heterogeneity, different severity levels of atopic dermatitis may produce some bias. Thirdly, no obvious increase in serious adverse events was seen after the 12-week treatment with abrocitinib, and longer follow-up should be conducted to confirm its safety.