Introduction
Psoriasis has become one common immune-mediated inflammatory disease, and its treatment is still challenging [1–5]. These patients often desire complete skin clearance and improved long-term efficacy [6–8]. In patients with psoriasis, the scalp is the most commonly affected area, and approximately 80% of psoriasis patients suffer from scalp involvement [9]. These patients have significantly reduced mental health, quality of life and social functioning [10, 11].
Topical therapies are regarded as the first-line therapeutic option for treatment of scalp psoriasis, but may provide inadequate relief for patients with moderate to severe scalp psoriasis [10, 12, 13]. These patients need systemic or biologic agents with frequent monitoring for adverse events [14, 15]. As an oral phosphodiesterase 4 inhibitor, apremilast has shown some efficacy in patients with psoriasis, including in subsets of patients with scalp involvement [16–19].
Recently, several studies have compared the efficacy of apremilast for psoriasis, but the common use of apremilast for psoriasis has not been well established [16, 17, 20, 21]. The usage of this medicine is as follows: Day 1: 10 mg in the morning, Day 2: morning 10 mg and evening 10 mg, Day 3: morning 10 mg and evening 20 mg, Day 4: morning 20 mg and evening 20 mg, Day 5: morning 20 mg and evening 30 mg, Day 6 and thereafter: 30 mg twice daily [21].
Material and methods
This systematic review and meta-analysis were performed based on the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement and Cochrane Handbook for Systematic Reviews of Interventions [22, 23]. No ethical approval and patient consent were required because all analyses are based on previous published studies.
Literature search and selection criteria
We have systematically searched several databases including PubMed, Embase, Web of science, EBSCO, and the Cochrane library for RCTs published from inception to February 2022 with the following keywords: “apremilast” AND “psoriasis”. The reference lists of retrieved studies and relevant reviews were also hand-searched and the above process was performed repeatedly in order to include additional eligible studies.
The inclusion criteria were presented as follows: (1) study design is RCT, (2) patients are diagnosed as psoriasis, and (3) intervention treatments are apremilast versus placebo.
Data extraction and outcome measures
Some baseline information was extracted from the original studies, and they included the first author, number of patients, age, body mass index (BMI), duration of psoriasis and detailed methods in two groups. Data were extracted independently by two investigators, and discrepancies were resolved by consensus. We have contacted the corresponding author to obtain the data when necessary.
The primary outcomes were PASI-75 (LOCF) and PASI-75 (NRI). Secondary outcomes included sPGA response (LOCF), sPGA response (NRI), PASI-50 (LOCF), PASI-90 (LOCF), adverse events and serious adverse events.
Quality assessment in individual studies
The methodological quality of each RCT was assessed by the Jadad Scale which consists of seven evaluation elements: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points) [24]. One point would be allocated to each element if they have been conducted and mentioned appropriately in the original article. The score of Jadad Scale varies from 0 to 5 points. An article with Jadad score ≤ 2 is considered to be of low quality. The study is thought to be of high quality if Jadad score ≥ 3 [25].
Statistical analysis
We assessed the odd ratio (OR) with 95% confidence interval (CI) for all dichotomous outcomes. Heterogeneity was evaluated using the I2 statistic, and I2 > 50% indicated significant heterogeneity [26]. The random-effects model was used for all meta-analyses. We searched for potential sources of heterogeneity for significant heterogeneity. Sensitivity analysis was performed to detect the influence of a single study on the overall estimate via omitting one study in turn or performing the subgroup analysis. Owing to the limited number (< 10) of included studies, publication bias was not assessed. Results were considered as statistically significant for p < 0.05. All statistical analyses were performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
Results
Literature search, study characteristics and quality assessment
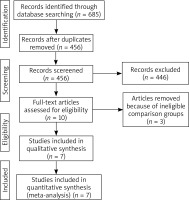
Figure 1 showed the detail flowchart of the search and selection results. Six hundred eighty-five potentially relevant articles were identified initially. Finally, seven RCTs were included in the meta-analysis [16–21, 27].
The baseline characteristics of seven included RCTs were shown in Table 1. These studies were published between 2013 and 2020, and the total sample size was 2173. Among the included RCTs, apremilast was administered at a dose of 20 mg or 30 mg twice daily for 16 weeks.
Table 1
Characteristics of included studies
Among seven included RCTs, four studies reported PASI-75 (LOCF) [16, 17, 21, 27], three studies reported PASI-75 (NRI) [16, 17, 27], four studies reported sPGA response (LOCF) and sPGA response (NRI) [16, 17, 20, 27], four studies reported PASI-50 (LOCF) [16, 17, 21, 27], three studies reported PASI-90 (LOCF) [16, 17, 27], five studies reported adverse events [16, 17, 19, 20, 27] and six studies reported serious adverse events [16, 17, 19–21, 27]. Jadad scores of the seven included studies varied from 4 to 5, and all seven studies have high quality based on the quality assessment.
Primary outcomes: PASI-75 (LOCF) and PASI-75 (NRI)
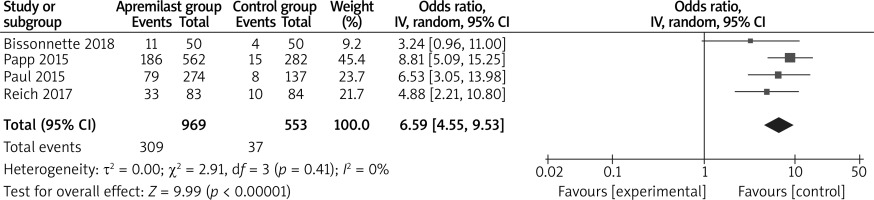
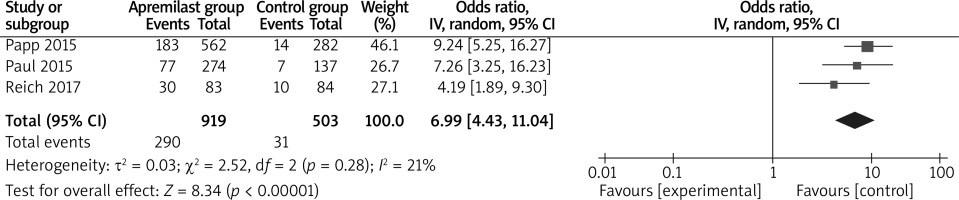
The random-effects model was used for the analysis of primary outcomes. The results found that compared to placebo for psoriasis, apremilast was associated with improved PASI-75 (LOCF) (OR = 6.59; 95% CI: 4.55 to 9.53; p < 0.00001) with no heterogeneity among the studies (I2 = 0%, heterogeneity p = 0.41, Figure 2) and PASI-75 (NRI) (OR = 6.99; 95% CI: 4.43 to 11.04; p < 0.00001) with low heterogeneity among the studies (I2 = 21%, heterogeneity p = 0.28, Figure 3).
Sensitivity analysis
There was no significant heterogeneity for primary outcomes, and thus we did not perform sensitivity analysis by omitting one study in each turn.
Secondary outcomes
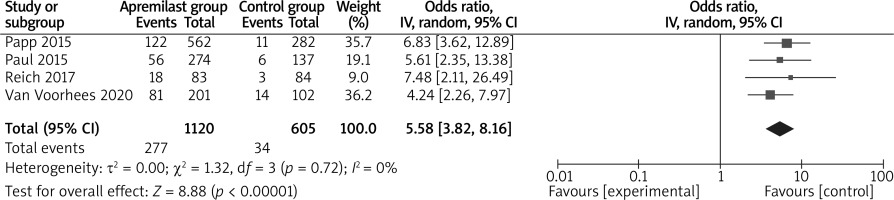
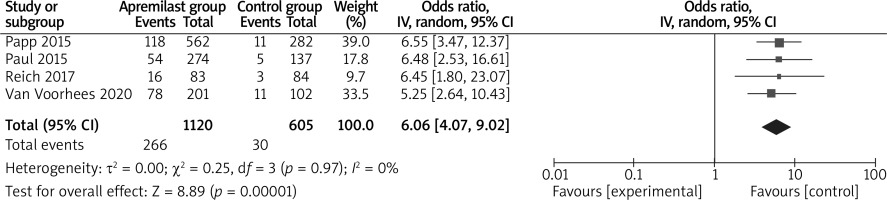
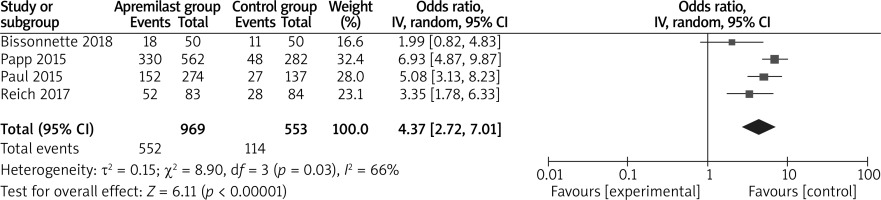
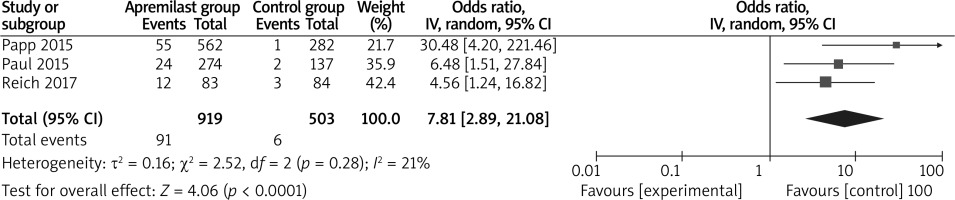
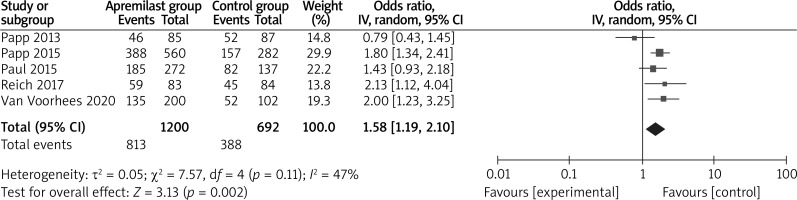
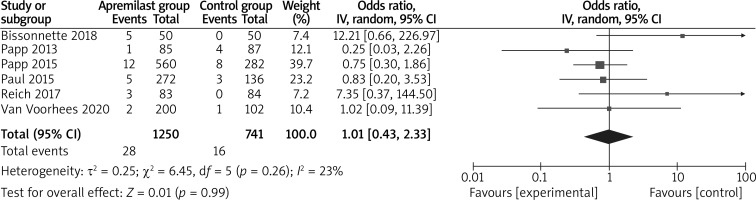
In comparison with placebo for psoriasis, apremilast resulted in the obvious increase in sPGA response (LOCF) (OR = 5.58; 95% CI: 3.82 to 8.16; p < 0.00001; Figure 4), sPGA response (NRI) (OR = 6.06; 95% CI: 4.07 to 9.02; p < 0.00001; Figure 5), PASI-50 (LOCF) (OR = 4.37; 95% CI: 2.72 to 7.01; p < 0.00001; Figure 6), PASI-90 (LOCF) (OR = 7.81; 95% CI: 2.89 to 21.08; p < 0.0001; Figure 7), adverse events (OR = 1.58; 95% CI: 1.19 to 2.10; p = 0.002; Figure 8), but showed no increase in serious adverse events (OR = 1.01; 95% CI: 0.43 to 2.33; p = 0.99; Figure 9).
Discussion
Psoriasis often causes intense pruritus. For instance, scalp psoriasis can be a very distressing manifestation of psoriasis [10, 28]. Many systemic drugs such as etanercept and secukinumab have been developed to treat moderate to severe plaque psoriasis of the scalp in phase 3 studies of patients [29, 30]. Especially, apremilast treatment was associated with significantly greater improvements in scalp psoriasis, scalp and whole body itch, and quality of life compared with placebo [17, 27].
Our meta-analysis confirmed that apremilast was able to produce significantly better treatment efficacy than control intervention for psoriasis, which was supported by the improvement in PASI-75 (LOCF), PASI-75 (NRI), sPGA response (LOCF), sPGA response (NRI), PASI-50 (LOCF) and PASI-90 (LOCF). However, we found the increase in adverse events after apremilast treatment in psoriasis patients. These adverse events mainly included diarrhoea, nausea, headache, vomiting and agitation. They were generally acceptable and tolerant [16, 17, 21, 27]. Therefore, this meta-analysis revealed no increase in serious adverse events after apremilast treatment.
Regarding the sensitivity analysis, although no significant heterogeneity remained for the primary outcomes, several factors may produce some bias. Firstly, the apremilast was administered at a dose of 20 mg or 30 mg twice daily, and different doses of apremilast may produce some heterogeneity. Secondly, various kinds of psoriasis were included in this meta-analysis, including plaque psoriasis of the scalp and palmoplantar psoriasis. Thirdly, different duration of psoriasis history may produce some impact on the efficacy assessment of apremilast.
Several limitations exist here. Firstly, our analysis was based on only seven RCTs, and more RCTs with a larger sample size should be conducted to explore this issue. Next, different doses of apremilast and various kinds of psoriasis were included, which may generate some bias. Finally, ideal methods for apremilast remain elusive.