Introduction
Small airways play an important role in asthma as well as in its severe phenotype and during the exacerbations. This is proven by numerous publications [1–4]. In our previously published work we showed that extra-fine ciclesonide provides a more potent anti-inflammatory effect than standard particle inhaled corticosteroids (ICS) [5].
William van Aalderen published a study in which he showed that small-particle ICS cause better prevention of exacerbations (manifested as a lower number of hospitalizations and exacerbations) and better asthma control than standard size particle ICS. Small-particle ICS were no worse in preventing exacerbations than a step-up long-acting β2-agonists (LABA)/ICS therapy [6].
Similar results concerning adult asthmatic patients have been published by E. Israeli. Small particle ICS have similar efficacy in exacerbation prevention to LABA/ICS combination treatment in step-up therapy [7].
Small airways play an important role in unstable and severe asthma pathogenesis [8]. Extra-fine ICS seem to penetrate to peripheral airways and produce a more potent anti-inflammatory effect in patients with small airways involved [9, 10].
Despite a significant progress in diagnostics and treatment of asthmatic patients within the natural course of the disease, exacerbations may occur. Progressing deterioration of the lung function, dyspnoea, cough, wheezing and chest tightness are the main symptoms of the asthma exacerbation. The first step in the prevention of severe asthma exacerbations is the intensification of the anti-inflammatory treatment with high doses of inhaled corticosteroids [11].
Aim
The aim of the study was to assess the efficacy of extra-fine ciclesonide (CIC) in patients who have been losing control of their asthma despite being treated with medium doses of standard size-particle inhaled corticosteroids and LABA as the second controller.
Material and Methods
Patients
Seventy-four patients who had been losing control of their asthma and/or had had mild exacerbation were included. Those patients had been treated with 500 μg of fluticasone or 640 μg of budesonide per day until the beginning of the study. Clinical criteria for qualification included an increase in the frequency of day and night-time asthma symptoms, an increase in short acting β-agonist (SABA) use, a decrease in Asthma Control Test (ACT) score, a confirmation of clinical worsening of the disease proven by a decrease in the forced expiratory volume in 1 s (FEV1) value. Patients requiring antibiotic therapy or fulfilling the criteria of severe asthma exacerbation during the baseline visit were not included.
Study schedule
The study period was 14 days. The clinical assessment, FENO and spirometry were performed on day 1, 7 and 14 of the study. The clinical evaluation included: Asthma Control Test, day (0 – 5 points) and night (0 – 4 points) symptoms score, rescue medication consumption (salbutamol 100 μg MDI), the necessity of oral corticosteroids (OCS) or antibiotics treatment, adverse events (AE) and resolution of asthma exacerbation (Table 1).
Table 1
The scheme of the protocol
Subjects received the following anti-inflammatory interventions: high doses of CIC 1280 μg (group A), CIC 640 μg added to the current dose of ICS (group B) or a doubled dose of current ICS (group C). The LABA were continued in the same dose, SABA were used as a rescue medication.
This was an open study, performed without randomization. Patients were consecutively assigned to each of the study arms.
Asthma was diagnosed according to the criteria recommended by the GINA [11]. Asthma patients were non-smokers and during the last year were not exposed to passive smoking.
The study protocol was approved by the Ethics and Research Committee of the Medical University of Bialystok, number R-I-002/455/2014. Informed consent form was obtained from every patient entering the study.
Measurements
Exhaled nitric oxide (FENO) was measured with Sievers 280i NO Analyzer (Boulder, Colorado, USA), which uses a chemiluminescence technique. The measurements were performed at an expiratory flow of 50 ml/s according to ATS recommendations for on-line measurement of FENO in adults [12].
The spirometry (FEV1) was performed using a MasterScreen Pneumo PC spirometer (Jaeger, Hoechberg, Germany), according to the ATS standards [13].
The study was conducted between September 2014 and September 2015.
Statistical analysis
An analysed data set contained qualitative and quantitative variables that were not normally distributed. For this reason only nonparametric tests that do not require Gaussian data distribution were applied. The computational tasks consisted of an analysis of the dependence between two qualitative variables, an analysis of two quantitative variables, and comparison of independent and dependent groups described by the quantitative data. The relations between qualitative variables were analysed using the χ2 test based on frequency tables. An investigation of the dependence between quantitative variables was performed using the Spearman correlation rank test. Independent groups of patients were compared using the Kruskal-Wallis ANOVA and the Mann-Whitney tests, groups 3 and 2, respectively. An analysis of dependent groups of patients was performed through the application of the Friedman ANOVA test. All calculations were performed using the Statistica 9 package.
Results
Characteristics of patients are presented in Table 2. We did not observe any significant differences in patient age, asthma history, laboratory results, spirometric parameters and FENO between particular treatment options.
Table 2
Characteristics of patients according to particular treatment options
Both ciclesonide treatment arms have shown statistically and clinically important advantages in comparison to the group where medium doses of previously used inhaled corticosteroid were doubled. Data are presented in Table 3 and in Figures 1–4.
Table 3
Changes in studied parameters between particular treatment options during the therapy period
Table 4
The analysis of the endpoints of this study
| Parameter | Group A (n = 25) | Group B (n = 25) | Group C (n = 24) |
|---|---|---|---|
| Antibiotics treatment | 3 | 5 | 6 |
| OCS treatment | 0 | 1 | 5 |
| Adverse events | 0 | 1 | 4 |
| End of exacerbation | 21 | 19 | 6 |
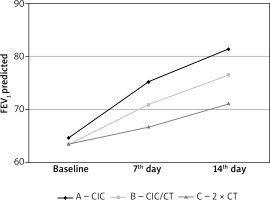
On day 7 and 14 of the treatment we observed statistically significant improvement of FEV1 among all groups, however, the change in the group treated with high doses of ciclesonide (group A) was significantly higher and appeared earlier than in other study groups (Table 3, Figure 1).
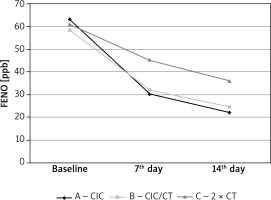
A decrease in FENO value between baseline and day 14 was statistically significant for all treatment options. However, the differences between the studied groups of patients were not statistically significant. The dynamics of the decrease in FENO for both groups treated with ciclesonide (group A and B) were statistically significantly higher over the use of a high dose of non-extra fine ICS. Both FENO decrease and its dynamics were statistically correlated with eosinophilia, IgE and presence of atopy. Higher eosinophilia, IgE and in case of atopy, a higher baseline and higher decrease in FENO during the therapy was observed (Table 3, Figure 2).
Figure 2
Changes in exhaled nitric oxide concentrations during therapy depending on selected treatment methods
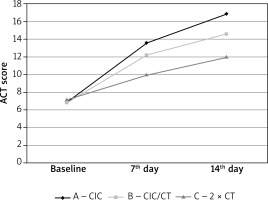
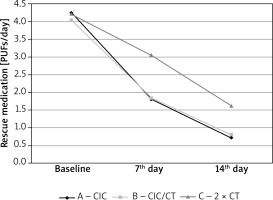
The average value of Asthma Control Test (ACT) for particular treatment days is presented in Figure 3 and in Table 3. All three evaluated treatment methods significantly improved the degree of asthma control in comparison to baseline. Patients treated with high doses of ciclesonide (group A) showed a statistically higher and more dynamic increase in their ACT score (Table 3, Figure 3). All treatment strategies caused a significant reduction in rescue medication consumption. Treatment options with ciclesonide (group A and B) showed significant therapeutic advantages over using a high dose of non-extra fine ICS (Table 3, Figure 4).
At the baseline we did not observe statistically significant differences in day and night symptoms score between particular treatment options. On day 7 and 14 of treatment we observed a significantly higher reduction in daytime symptoms score for both treatment arms with ciclesonide in comparison to the one using a high dose of non-extra fine inhaled corticosteroids. Night-time symptoms scores both on day 7 and 14 were statistically significantly lower in both CIC treatment arms (Table 3).
A comparison of groups A and B shows a greater response among patients treated with higher doses of ciclesonide (group A) (Table 3).
We evaluated how often patients (depending on particular therapeutic options) required antibiotics or oral steroid treatment, adverse events frequency (connected with inhaled corticosteroid treatment) and the number of patients whose exacerbation/loss of control had been resolved after 14 days of therapy.
Patients in groups treated with ciclesonide presented statistically significant fewer cases where OCS or antibiotic therapy were necessary. Moreover, asthma exacerbation time among these patients was significantly shorter. An analysis of the endpoints of this study has been presented in Table 4.
Discussion
Small airways play a significant role in unstable and severe asthma pathogenesis [14–16]. Extra-fine ICS seem to penetrate to peripheral airways and present a more potent anti-inflammatory effect in patients with small airways involved. Extra-fine ICSs are effective in achieving asthma control and present an effect which is no worse than adding LABA to therapy [6].
Despite a significant progress in diagnostics and treatment of asthmatic patients, exacerbations may occur as a natural course of the disease. Progressing deterioration of lung function, dyspnoea, cough, wheezing and chest tightness are the main features of asthma exacerbation. The first step in prevention of severe asthma exacerbations is intensifying the anti-inflammatory treatment with high doses of ICS [11].
The aim of the study was to assess the efficacy of extra-fine ciclesonide in patients losing control of their asthma despite being treated with medium doses of standard size-particle ICS and LABA as the second controller.
Treating with high doses of ciclesonide brought a quick and potent clinical improvement along with the proper safety profile in patients suffering from mild asthma exacerbation (or who had been losing asthma stability). Therapeutic options of using ciclesonide (especially in high doses) showed a significant therapeutic advantage over using high doses of non-extra fine ICS. High doses of extra-fine ciclesonide allowed decreasing the need for interventional OCS therapy or/and antibiotics without additional safety concerns. Treatment with small-particle ICS (CIC) gives the opportunity to avoid the development of full-scale exacerbation (in clinical picture) and the need for OCS short-term therapies.
A possible reason for the strong anti-inflammatory effect of ciclesonide may be the solution formulation for HFA-powered MDI with a small particle size that extends and reaches the peripheral airways, which are important sites of significant inflammation in asthma patients [17]. Ciclesonide also has low oropharyngeal and high pulmonary deposition resulting in a high therapeutic index and low potential for adverse events [18]. Activation of a potent metabolite with a high affinity for pulmonary glucocorticoid receptors which provides anti-inflammatory activity at the desired target site is also very important [19]. Fatty acid conjugation of the active metabolite results in a depot effect that prolongs the anti-inflammatory action [20].
The safety of ICS, especially when using high doses, depends on their dose, formulation and pharmacological properties. Marczak published a study in which he showed that switching from prednisone to very high doses of ciclesonide normalized the hypothalamic-pituitary adrenal axis function and also improved the disease control and the lung function in patients with difficult asthma [21].
Extra-fine ICS updosing – adjustment should be a treatment of first choice in achieving satisfactory asthma control and prevention of the imminent exacerbation in practice. It is connected with anti-inflammatory properties and the ability to penetrate to small airways affected by the inflammatory process.
The possible limitations of the study are small groups of patients and a lack of randomization.
Conclusions
The original aspect of the study is to show that ciclesonide, which is safe and effective in asthma treatment, also presents a rapid clinical improvement and a potent anti-inflammatory effect in comparison to non-extra fine ICS in patients with mild asthma exacerbation/loss of control. There is an interesting strategy to apply two inhaled corticosteroids having different pharmacological properties and different deposition into the airways at the same time.
More clinical studies for assessing the long-term efficacy of ciclesonide in comparison with non-extra fine inhaled corticosteroids in preventing asthma exacerbations and maintaining asthma control are needed.
Conflict of interest
Ziemowit Zietkowski and Anna Bodzenta-Lukaszyk have received payments for lectures from Takeda and AstraZeneca. The other authors declare that they have no conflict of interest in the publication of this manuscript. This work was supported by research grant No 3-35523P from the Medical University of Bialystok, Poland.