Introduction
Scleroderma (Sc) is a connective tissue disease whose pathogenesis is still unknown. There are two main types of Sc, localized and systemic. Systemic sclerosis (SSc) consists of limited cutaneous systemic sclerosis (previously known as the CREST syndrome) and diffuse cutaneous SSc. Systemic sclerosis is associated with internal organ involvement and increased mortality. The classification of SSc is based on skin involvement [1]. Limited cutaneous systemic sclerosis (LcSSc) is associated with skin thickening distal to the elbows, distal to the knees, and/or face without trunk involvement. Diffuse cutaneous systemic sclerosis (DcSSc) is associated with skin thickening that can involve skin proximal to the elbows, proximal to the knees, face, and/or trunk. Internal organs usually affected by scleroderma are the gastrointestinal tract, lungs, kidneys, skeletal muscle, and pericardium [2–4].
The Rodnan skin score (RSS) is a method of assessing skin involvement among patients with SSc. The RSS is an established method of examining skin thickness in SSc patients. It is a semiquantitative, non-invasive, rapid method to measure skin thickness with high reproducibility [5]. Thus, it is widely used both in clinical trials and clinical practice. Previous studies have investigated the relationship between the incidence of disease-related events or the presence of organ involvement and skin thickness that was quantitatively evaluated [6–10]. Skin thickening is a characteristic feature of SSc. The more extensive a patient’s skin involvement is the more severe the internal organ manifestation(s), the poorer the prognosis, and the greater the disability.
The modified Rodnan Skin Score (mRSS), introduced in 1979, is a common method for assessing the severity of skin involvement [6]. It is a palpation-based, semi-quantitative score used in the quantitative estimation of skin hardening measured in 17 areas of the body, contrary to classic RSS (cRSS) in which 20 body areas are checked. Each examined area is assessed on a 4-point scale from 0 – representing no skin hardness, to 3 – representing severe skin thickness (inability to make the skin fold between two fingers). Each area is added together to give an overall result ranging from 0 (no hardening) to 51 (severe hardening of the skin in all 17 areas). The RSS score divides the severity of skin lesions into: mild (1–14), moderate (15–29), severe (30–39), or terminal (≥ 40).
The mRSS is an established factor influencing the prognosis due to its accurate reflection of skin biopsy thickness in SSc [6]. Studies have shown that the severity of skin hardening assessed using the mRSS was predictive for disease outcome [7]. The limitations of using the mRSS include examiner’s experience and skills as well as difficulties in standardization among different centres [11]. Moreover, the mRSS may not be sensitive enough to detect small but relevant (subclinical) changes in skin thickness over time [12]. Nevertheless, the mRSS is preferred among clinicians as a non-invasive and relatively cheap method [11]. Due to the limitations of the mRSS, an objective and sensitive method of skin assessment in SSc is still under investigation. Several non-invasive methods for quantifying skin involvement in SSc have been described over the past 10 years [11–13]. Ultrasound (US) skin imaging, high-frequency B-mode ultrasonography, ultrasound shear-wave elastography (US-SWE), acoustic radiation force impulse (ARFI) imaging, and magnetic resonance imaging are frequently considered tools for the skin assessment in SSc. However, ultrasound has yet to be confirmed as a reliable method of skin involvement imaging in SSc. It is also required to establish the standard operating procedure of patient examination to make sure that the new tool is quick, repeatable, and easy to learn.
Aim
This study aims to verify whether elastography is a reliable method to examine SSc progression and possibly provide one useful place to examine to be an easy, cheap, and reliable examination tool.
Material and methods
The study was conducted between 31 October 2018 and 23 February 2019, at the Clinic of Dermatology, Centre of Postgraduate Medical Education, Central Clinical Hospital of the Ministry of the Interior, Warsaw, Poland.
Study participants
Forty patients with a confirmed diagnosis of systemic sclerosis based on the ACR and EULAR classification criteria were recruited to participate in the study (either with dSS or lSSc) [14]. Out of the 40 patients with SSc, 34 (85.0%) patients were female (F) and 6 (15.0%) were male (M); F/M ratio was 4:1. The age of the patients ranged from 23 to 77 years (mean: 44.4 ±13.5 years). Twenty-nine (72.5%) of the patients were diagnosed with lSSc and 11 (27.5%) with dSSc. The median time since the diagnosis of SSc was 9 years (range: 0–30 years). The control group consisted of 28 healthy sex- and age-matched individuals, 23 (82.1%) of them were female and 5 (17.9%) were male, aged between 22 and 71 years old (mean: 44.9 ±14.4 y.o.). All examined SSc patients (n = 40; 100.0%) and 25 (89.3%) healthy controls used their right hand as the dominant extremity. In both groups, the co-existence of other autoimmune or skin diseases was used as an exclusion criterion to eliminate the influence of other phenomena on the elastography results. The study protocol was approved by the Institutional Bioethics Committee. All patients and healthy volunteers provided informed consent for participation in the study.
Skin assessment
The skin thickness in patients with SSc was expressed using the classic RSS based on the palpation of 20 cutaneous sites: face, neck, anterior chest, abdomen, upper and lower back, right and left upper arm, right and left forearm, right and left hand, a finger of the right and left hand, right and left thigh, right and left leg, right and left foot. All the anatomical sites used for the RSS evaluation were also assessed with SWE. Each anatomical site was examined separately, with the individual in a supine or prone position. Ultrasonographic scans were obtained with a Toshiba Aplio i900 ultrasound machine (2019 Malaysia) using a 5–18 MHz transducer. Elastography results (strain) were obtained and expressed as previously described by Sobolewski et al. [15].
Statistical analysis
In order to analyse the relationships between the studied variables, multiple regression and cluster analysis (k-means method) were used. The level of statistical significance was set at p < 0.05. All calculations were performed using Statistica version 13.3 software (2017; TIBCO Software Inc.).
Results
Upon analysis, the existence of 3 clusters of examined body areas emerged within the SSc group and control group. Descriptive statistics and distances from the centre of the cluster are presented in the Tables 1–4 and Figures 1 and 2.
Table 1
Cluster elements and distances from the centre of the cluster; table for the three separate clusters in the SSc group
Table 2
Cluster elements and distances from the centre of the cluster; table for the three distinguished clusters in the control group
Table 3
The regression analysis of the results of elastography examination in different areas of the body in correlation to the results of the Rodnan skin score (researcher no. 1)
Table 4
The regression analysis of the results of elastography examination in different areas of the body in correlation to the results of the Rodnan skin score (researcher no. 2)
Figure 1
The results of cluster analysis using the k-mean elastography results in the studied areas of the body in the SSc group
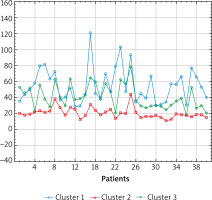
Figure 2
The results of cluster analysis using the k-mean elastography results in the examined body areas in the control group
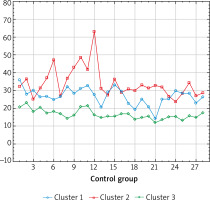
The highest mean results in the control group were obtained in the right and left foot and leg. The highest mean test results in the study group were obtained in the distal parts of the body: fingers of the right and left hand, right and left hand, and the right and left foot, which is consistent with tests performed by other methods.
The regression analysis showed that the elastography examination in the right-hand finger and right forearm had a statistically significant influence on increased Rodnan scores (carried out by the first researcher). The regression analysis also showed that elastography in the right-hand finger and left hand had a statistically significant influence on increased Rodnan scores (when performed by the second researcher).
Discussion
During the last decade, the applicability of US testing among patients with SSc has been researched to find a tool for the assessment of skin involvement [15–24]. Several studies have shown that US-SWE can be used to quantitatively characterize the degree of skin involvement among patients with systemic sclerosis [15, 20, 21].
Li et al. suggested the feasibility of assessing skin involvement in SSc with skin ultrasound imaging. This study showed that echogenicity of skin correlated with skin thickness and the local mRSS. The results obtained by Li et al. [22] indicated that the ultrasound-measured skin thickness parameter was more sensitive than the mRSS in detecting skin involvement in SSc patients.
Other studies have suggested that US examination of only one anatomical site such as the proximal phalanx of the finger might reflect local skin involvement assessed by palpation and the overall skin involvement [16, 22–24].
According to Naredo et al. [24], US examination allows for a detailed image of the skin layers allowing for a reliable measurement of dermal thickness in SSc patients. These authors have suggested that ultra-high-frequency ultrasound was a tool that could provide a precise identification and measurement of dermal thickness. Moreover, they reported that dermal thickness in the finger is significantly higher in patients with SSc than the controls while in the forearm it was significantly lower in patients with SSc than the controls [24].
We argue that because mRSS may not be sensitive enough to detect slight but relevant changes in skin thickness over time [12], SWE should at least complement if not be a substitute for the mRSS.
Conclusions
In this study, the authors have shown that elastography is a useful method for the measurement of skin thickness in patients with SSc. The mean values obtained in the areas indicated in the results’ description allow for the conclusion that these areas of the body are the most important for diagnosing and determining the severity of negative symptoms. Moreover, elastography is a reliable method of determining skin involvement in patients with systemic sclerosis and the results correlate positively to the RSS. That correlation was confirmed by the results indicating that both researchers showed a significant influence of elastography on the Rodnan test when examining the finger of the right hand. These results in the right-hand finger can be treated as an important diagnostic indicator and predictor for the severity of the negative symptoms associated with SSc. Both statistical analyses confirmed the reliability of the SWE examination method in patients with SSc and identified predictive body areas.
Elastography examination is also a dependable method of assessing the progression of SSc. High elastography scores correlate with a high RSS. In the authors’ opinion, this study suggests that elastography is a reliable method to examine SSc progression and provides one useful site of examining allowing it to be an easy, cheap, and repeatable examination tool. The results of this study overlap and show the reliability of elastography as a method of assessing skin involvement. However, there is a need for an additional multicentre study to validate the results by independent physicians and check the compatibility of their outcomes. The use of US in the evaluation of the skin can allow for earlier diagnosis and better disease status monitoring. There is a need for the development of a standard operating procedure of examination for physicians treating patients with SSc. Despite the limitations of this study such as a small treatment group and only two specialists performing the research, to the best of our knowledge it is the first study to compare the cRSS with SWE and test the reliability of SWE as a method of skin assessment among SSc patients. The next step in the research process should be a multicentre research project with the participation of a larger group of specialists. The authors of this study believe that SWE skin assessment can be a sensitive testing method to detect skin involvement in SSc patients. The widespread availability of US machines could make this the preferred method used by clinicians as it is a non-invasive and relatively cheap method.







