Introduction
Autoimmune and inflammatory diseases are a common dermatological and psychological problem [1]. With the development of the pharmaceutical industry, as well as the lack of effectiveness of conventional therapeutic methods, new and better therapeutic agents are sought. These drugs are designed to ease the effects of autoimmune diseases or to stop the spread of inflammation [2]. There are many agents, including biological drugs, e.g. adalimumab, etanercept and ustekinumab which are the subject of our study.
The clinical picture of inflammatory diseases differs depending on the system covered.
However, a common feature of inflammatory diseases is the existence of common signalling pathways (mainly related to apoptosis, the cell cycle) due to the abnormal or increased production of pro-inflammatory cytokines. Therefore, blocking of some signalling pathways may contribute to the improvement of the patient’s health. This concerns mainly processes related to apoptosis. The interaction of pro-inflammatory cytokines with many of the body’s receptors can cause effects at the molecular level. The effect may be an altered expression that results in disease progression [3, 4].
One of these autoimmune diseases is psoriasis. It occurs mainly due to genetic predisposition, immunological factors, and altered activity of pro-inflammatory cytokines. In predisposed people, its occurrence can be initiated or exacerbated by environmental factors, such as infections, stress, and certain medications. However, the root cause of psoriasis is not exactly known. Furthermore, autoimmunity is not the only component of psoriasis development [5, 6]. The inflammatory reaction manifested by the interaction of inflammatory cells and mediators with skin cells and other organs is a key element involved in its pathogenesis [7]. The huge role of overactive T lymphocytes, especially the Th1 and Th17 subpopulations, as well as the overproduction of some pro-inflammatory cytokines (e.g., IL-6 – interleukin 6, IL-8 – interleukin 8, IL-12 – interleukin 12, IL-23 – interleukin 23), secreted by both cells of the immune system and epidermal keratinocytes is emphasized [8].
The pathophysiology of many inflammatory disorders is largely based on the increased production of inflammatory cytokines by the patient’s immune system [9]. The use of drugs whose action is based mainly on blocking specific cytokines involved in the cascades of inflammatory reactions becomes justified [10]. Therefore, an important element of psoriasis therapy is to learn about the individual cytokines that are responsible for its pathogenesis and to find a drug that, by blocking their activity, will improve the patient’s condition or completely cure it.
Currently, this function is performed mainly by biological drugs, including the ustekinumab – a fully human IgG1κ monoclonal antibody. Ustekinumab inhibits the bioactivity of human IL-12 and IL-23 by preventing p40 from binding to the receptor the IL-12Rβ1 protein found on the surface of cells of the immune system. By binding to the split p40 subunit of IL-12 and IL-23, ustekinumab may exhibit its clinical effects in psoriasis, psoriatic arthritis, Crohn’s disease and ulcerative colitis. Ustekinumab also indirectly affects other cytokine downstream of the inflammatory pathway. They include among others IL-6 and IL-8, which are involved in inflammatory reactions with a predominance of Th17 lymphocytes, as well as interferon γ (IFN-γ), in turn enhancing the inflammatory response from Th1 lymphocytes [11].
Aim
The aim of the study was to evaluate the expression profile of genes related to the inflammatory response – IL-6, IL-8 and IFN-γ – in monitoring ustekinumab therapy in patients with psoriasis vulgaris.
Material and methods
The test material consisted of peripheral blood mononuclear cells (PBMCs) from the blood of 14 patients (10 men and 4 women, aged 48 ±10 years) suffering from psoriasis in the Polish population, who were undergoing ustekinumab therapy. The blood samples used in this study were obtained from patients four times: immediately before the first drug administration (0 weeks) and 16, 28, and 40 weeks after drug administration. Ustekinumab was the first biological drug to be used in the treatment of these patients; conventional treatments for psoriasis that had previously been used in patients turned out to be ineffective. The drug was administered as a subcutaneous injection at a dose of 45 mg; the first 2 doses every 4 weeks, then every 12 weeks. The inclusion and exclusion criteria were described previously by Grabarek et al. [12].
Each time before and 2 h after ustekinumab administration, blood was collected for routine biochemical tests. On the day of drug administration, monitoring of psoriatic lesions was performed. The psoriasis area and severity index (PASI) and body surface area (BSA) coefficients were determined; their values are given in Table 1. Additionally, the assessment of the quality of life of patients undergoing treatment was carried out using the Dermatology Life Quality Index (DLQI) questionnaire.
Table 1
PASI, DLQI and BSA values for patients undergoing the study
| Indicator | Time [weeks of therapy] | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| PASI | 0 | 26 | 5 | 19 | 35 |
| 16 | 8 | 6 | 2 | 16 | |
| 28 | 5 | 2 | 2 | 7 | |
| 40 | 4 | 2 | 1 | 6 | |
| DLQI | 0 | 20 | 5 | 14 | 27 |
| 16 | 7 | 4 | 1 | 13 | |
| 28 | 8 | 4 | 0 | 13 | |
| 40 | 6 | 3 | 1 | 8 | |
| BSA [%] | 0 | 54 | 10 | 41 | 65 |
| 16 | 24 | 20 | 5 | 56 | |
| 28 | 16 | 12 | 5 | 39 | |
| 40 | 6 | 3 | 3 | 9 |
Total RNA was extracted from specimens using a commercially available kit (Total RNA Prep Plus Kit, A&A Biotechnology, Gdynia, Poland) according to the manufacturer’s instructions. The quantitative and qualitative evaluation of the extracts was performed using a spectrophotometer (Maestrogen Inc., Hsinchu City, Xiangshan District, Taiwan) and the agarose electrophoresis method (1% agarose gel; Simply Safe reagent).
The expression of genes encoding IL-6, IL-8, and IFN-γ was assessed using the quantitative reverse transcriptase real-time PCR (RT-qPCR) method (i.e., quantitative DNA polymerase chain reaction), preceded by the transcription of RNA into cDNA using reverse transcriptase. The gene encoding β-actin was used as the reference gene (endogenous control). RT-qPCR assays were performed in triplicate for all samples.
The set of Sensi-Fast™ reagents (Bioline, London, UK) and primers (Forward and Reverse) specific for: IL-6, IL-8, IFN-γ, and ACTB were used (Table 2). Commercially available oligonucleotides were purchased from Sigma-Aldrich (St. Louis, MO, USA). The study also utilized the Opticon™ DNA Engine Sequence Detector (MJ Research Inc., Watertown, MA, USA).
Table 2
Characteristics of primers used for real-time RT-qPCR
| Gene | Sequence of primers | Length of amplicon [bp]a | Tm [°C]b |
|---|---|---|---|
| IL-6 | Forward: 5’GCAGAAAAAGGCAAAGAATC3’ Reverse: 5’CTACATTTGCCGAAGAGC3’ | 178 | 85.4 |
| IL-8 | Forward: 5’CTCTAACTCTTTATATAGGAATT3’ Reverse: 5’GATTGATTTTATCAACAGGCA3’ | 203 | 81.2 |
| IFN-γ | Forward: 5’GGTAACTGACTTGAATGTCC3’ Reverse: 5’TTTTCGCTTCCCTGTTTTAG3’ | 94 | 81.4 |
| ACTB | Forward: 5’TCACCCACACTGTGCCCATCTACGA3’ Reverse: 5’CAGCGGAACCGCTCATTGCCAATGG3’ | 295 | 88.2 |
The thermal profile for one-step RT-PCR was as follows: 45°C for 10 min for reverse transcription, 95°C for 2 min, 40 cycles at 95°C for 5s, 60°C for 10s, and 72°C for 5s. In order to assess the specificity of the RT-qPCR reaction, a melting-curve analysis of the reaction products was performed. The mRNA copy number of the studied genes was converted to 1 μg of total cellular RNA.
Statistical analysis
As part of the statistical analysis of the results, in the first place, the Shapiro-Wilk test was used to verify the normality of the distribution of the obtained data. To assess the differences in the number of mRNA copies of the studied gene in the case of normal distributions, the T-test for dependent samples was used. In the absence of normal distributions, non-parametric tests were used: Friedman ANOVA followed by the Wilcoxon pairwise test; the results for which p < 0.05 were considered significant. The Spearman rank-order correlation coefficient (rs ) was used to evaluate the relationships indicated by the data, and the results were considered to be statistically significant when the significance level was p < 0.05. All calculations were made in Statistica v.13.3 (Statsoft, Cracow, Poland).
Results
The transcriptional activity of all tested genes was found in peripheral blood mononuclear cells in all samples taken from patients, both before treatment (time T0) and at 16, 28, and 40 weeks of therapy.
A t-test was used to assess the differences in the number of IL-6 mRNA copies between measurement points. For the IL-8 and IFN-γ variables, the Friedman ANOVA test and the Wilcoxon pairwise test were used.
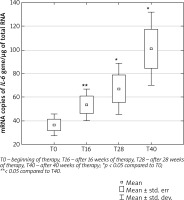
The number of IL-6 mRNA copies increased in the following weeks of treatment with ustekinumab, as shown in Figure 1. There was a statistically significant increase in the number of IL-6 mRNA copies after 28 and 40 weeks of therapy compared to time T0. There was also a statistically significant difference in IL-6 expression between weeks 16 and 40 of therapy.
Figure 1
Number of copies of mRNA/μg of total RNA of the IL-6 gene in PBMCs of psoriatic patients during ustekinumab therapy
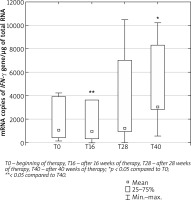
There was no statistically significant difference in the number of mRNA copies of the IL-8 gene in PBMCs of psoriatic patients during 40 weeks of ustekinumab therapy.
The number of IFN-γ mRNA copies increased in the following weeks of treatment with ustekinumab, as shown in Figure 2. There was a statistically significant increase in the number of IFN-γ mRNA copies after 40 weeks of treatment compared to time T0. A statistically significant difference in the expression of IFN-γ was also demonstrated between weeks 16 and 40 of therapy. The analysed dependencies are presented in Table 3.
Table 3
The differences in the number of IL-6, IL-8 and IFN-γ mRNA copies in the following weeks of treatment with ustekinumab
| Variables | Significance level (p) |
|---|---|
| IL-6: | |
| T0 vs. T16 | 0.063 |
| T0 vs. T28 | 0.022* |
| T0 vs. T40 | 0.001* |
| T16 vs. T28 | 0.338 |
| T16 vs. T40 | 0.017* |
| T28 vs. T40 | 0.107 |
| IL-8; | |
| T0 vs. T16 | 0.546 |
| T0 vs. T28 | 0.422 |
| T0 vs. T40 | 0.220 |
| T16 vs. T28 | 0.831 |
| T16 vs. T40 | 0.383 |
| T28 vs. T40 | 0.321 |
| IFN-γ; | |
| T0 vs. T16 | 0.804 |
| T0 vs. T28 | 0.109 |
| T0 vs. T40 | 0.009* |
| T16 vs. T28 | 0.081 |
| T16 vs. T40 | 0.005* |
| T28 vs. T40 | 0.447 |
Figure 2
Number of copies of mRNA/μg of total RNA of the IFN-γ gene in PBMCs of psoriatic patients during ustekinumab therapy
In the next stage of the study, the determined amounts of IL-6, IL-8 and IFN-γ mRNA/μg RNA were used to determine the relationship between them during treatment with ustekinumab. The relationship between the amount of mRNA of the studied genes and the values of PASI and BSA dermatological indicators at individual time points of ustekinumab treatment was also checked. The verification of the hypotheses about the existence of a relationship between the determined parameters was made using the Spearman rank correlation coefficient.
There were statistically significant (p < 0.05) correlations (rs) between the expression of the IFN-γ gene and changes in the level of IL-6 gene expression (rs = 0.68, p = 0.011; rs = 0.78, p = 0.001, respectively) as well as between the expression of the IFN-γ gene and the level of expression the IL-8 gene (rs = 0.587, p = 0.049 for both time points) at weeks 16 and 28 of ustekinumab treatment.
The clinical picture of patients with articular psoriasis changed in the following weeks of treatment with ustekinumab. It was observed that PASI value decreased over time during the treatment with ustekinumab. The greatest decrease was recorded up to week 16 of treatment. Similar relationships were also found in the case of the BSA [%] index. On the other hand, DLQI values in the next 16 weeks of treatment tend to decline, but between weeks 16 and 28 of therapy, they remain at a similar level, and then decrease again after 28 weeks. Correlations between the PASI index and the BSA index were recorded at weeks 16, 28 and 40 of ustekinumab treatment (rs = 0.86, p = 0.024; rs = 0.82, p = 0.034; rs = 0.81, p = 0.05, respectively for time points). It should also be noted that there is a statistically significant positive correlation between the number of IL-8 mRNA copies/μg RNA and the BSA index (rs = 0.94, p = 0.017) at week 40 of treatment.
Discussion
Changes in the concentration profile of certain cytokines, such as IL-12, IL-23, IL-17, IL-6, IL-8 and IFN-γ, play an important role in the pathology of psoriasis, and the understanding of their mutual correlations and molecular relationships is a necessity to find an effective form of therapy [13].
It should be emphasized that interleukins are pleiotropic cytokines that have a multidirectional effect on the cells of the innate and acquired immune system. The differentiation of the cells that produce them and the types of absorbing cells make it impossible to accurately determine the function of a given cytokine [14, 15]. Furthermore, in psoriasis interactions between different genes have been demonstrated, thus leading to release of various cytokines which can be regarded as the target of future effective therapy [16].
For this reason, more and newer molecular markers are still being sought to monitor response to the drug, regardless of the type of therapy used, as well as enabling early detection of insensitivity to the applied therapy [14].
Our studies assessed the expression profile of IL-8, IFN-γ and IL-6 in PBMCs patients with articular psoriasis treated with ustekinumab. We found that the number of IL-6 and IFN-γ mRNA copies increased in the following weeks of treatment with ustekinumab.
Our results confirmed previously published data that the Th1 cytokines (TNF-α, IFN-γ, and IL-12) and some proinflammatory cytokines (such as IL-6, IL-8, and IL-18) are influenced but in the serum of psoriatic patients. It is also known that expression of these genes is significantly increased in patients with other autoimmune diseases such as Crohn’s disease and multiple sclerosis [17, 18].
The studies conducted by Arican et al. [19] demonstrated a significant increase in IFN-γ, TNF-α, IL-6, IL-8, IL-12, IL-17 and IL-18 in the serum of psoriatic patients compared to the healthy controls [19]. The results of our research for IL-6, IL-8 and IFN-γ confirm the observations made by the above-mentioned authors, however, they were made with the use of other molecular methods, and the research material was PBMCs.
The JAK/STAT signalling pathway, which includes protein kinases as well as signal transducers and activators of transcription, is involved in the inflammatory process of psoriasis. Phosphorylation by STAT protein kinases enables their transport to the cell nucleus where they become transcription factors, thus influencing the expression of genes of various cytokines. This is an example of positive feedback, in which stimulation of a single signalling pathway affects the expression of other cytokines, stimulating inflammation and making it difficult to inhibit it [13].
A more significant role of the IL-23/Th17/IL-17 axis in the induction of autoimmune disorders, compared to the IL-12/Th1/IFN-γ loop, based on the example of autoimmune inflammation of the central nervous system of mice, has been experimentally proven by Murphy et al. [20]. They showed that reducing the expression of the gene encoding IL-23 may reduce the symptoms of disease, while inducing the expression of the gene encoding IL-12 exacerbated the disease. Mice with reduced expression of the IL-23 gene not only showed no symptoms of disease, but also became resistant to the development of autoimmune pathologies. In contrast, the RNA of mice lacking the expression of the IL-12 gene was characterized by the increased expression of IL-17, IFN-γ and other pro-inflammatory cytokines. The results suggest a significant contribution of IL-23 to the formation of autoimmune inflammation, while IL-12 mitigates the pro-inflammatory effects of IL-23 and mediates anti-inflammatory protection [20].
The increase in the concentration of some cytokines accompanying the development of their common feature has become a stimulus for searching for increasingly newer drugs, the action of which would mainly target the cascade of inflammatory reactions [21]. Biological drugs are examples of drugs that affect signalling and metabolic pathways. They regulate the activity of cytokines, the increased or abnormal production of which is the main cause of persistent inflammation in autoimmune diseases, including psoriasis [22].
This group of drugs includes ustekinumab, a monoclonal antibody with immunosuppressive and anti-inflammatory properties [23]. This drug, compared to conventional treatment methods, according to the latest studies conducted by the Psoriasis Longitudinal Assessment and Registry (PSOLAR) [24] in Caucasian patients, as well as studies conducted by the Programme for Europe-Asia Research Linkages (PEARL) in Asian patients [25], is characterized by a high safety profile and a low risk of side effects in the form of malignant tumours, cardiovascular diseases, and overall mortality. As with other biological drugs used in the treatment of autoimmune and inflammatory diseases, however, the immunosuppressive effects of treatment should be considered, which may lead to an exacerbation of comorbidities, in particular viral and bacterial infections, liver diseases, or circulatory failure [13]. Ustekinumab is a drug directed against two inflammatory interleukins, IL-12 and IL-23, but its effects also indirectly affect other cytokines downstream in the inflammatory cascade [26]. However, it is important not only to analyse one specific signalling pathway, but also to find links between different cascades of inflammatory reactions, activated by different cytokines, and to understand how they are regulated at the level of their gene expression [13].
In our study, the PASI index tended to decrease over the course of therapy. A similar tendency was shown by the BSA coefficient, which also decreased with each monitoring. Similar results were obtained in a study by Bissacotti-Steglich et al. [27]. They described a 53-year-old Brazilian with a 27-year history of severe psoriasis. Prior conventional treatment had no effect. This prompted the initiation of ustekinumab therapy. Before treatment, PASI and BSA indicated a high intensity of lesions (61.2% and 81%, respectively) and significant advancement of the disease. In the third week of treatment, both indices decreased (PASI to 3.8%, BSA to 13%), proving the effectiveness of the therapy [27].
The expression of some genes during ustekinumab therapy, which was tested using the RT-qPCR method, was also carried out by Gedebjerg et al. [28]. Contrary to the blood samples used in our study, biopsy samples of the diseased skin covered with plaques were used as material.
They reported that IL-6 and IL-8 expression was higher in the diseased skin compared to skin without lesions. Four monitoring activities were performed: before the first administration of the drug, after 4 days of drug use, after 4 weeks of drug use, and after 16 weeks of drug use. The expression of both genes declined over time, with the greatest decline at week 4 for the IL-6 gene and week 16 for the IL-8 gene. The duration of the study, which was 16 weeks, may have turned out to be too short to reliably show the differences in the expression of both genes. In the present study, the first statistically significant increase in IL-6 gene expression was found only after 28 weeks, and IFN-γ only after 40 weeks of the study, while the expression of the IL-8 gene did not change at all. For this reason, it may be assumed that the results obtained after 16 weeks by Gedebjerg et al. may not cover the entirety of the reactions that could be caused by ustekinumab therapy [28].
Moreover, the diseased epithelial tissue that was used for the study differs significantly from the molecular picture of the blood samples used in our study, and the inhibition of inflammation in the skin does not always indicate that it is inhibited in the blood. It is possible that this will become a stimulus to its intensification. As psoriasis is an inflammatory disease that can develop throughout the body, its pathogenesis should be investigated holistically and systemically rather than locally, focusing solely on skin lesions. In addition, any analysis of the action of biological drugs in the blood may contribute to a more detailed understanding of the mechanisms responsible for the development of resistance to the drug by the body, which is not possible thanks to studies performed on skin specimens. Monitoring pro-inflammatory markers in the blood may, therefore, be of greater clinical importance than tests carried out on the skin, and the sampling of material for testing itself is less burdensome for the patient than a biopsy.
The characteristic elevation of the level of pro-inflammatory cytokines (IFN-γ, IL-17A, TNF-α) and the reduction of the concentration of anti-inflammatory cytokines (IL-10 and IL-5) that occurs in psoriasis was also used in the study of the effects of ustekinumab (CNTO 1275) on PBMCs in the presence of IL-12 and IL-23 by Reddy et al. [29]. The study used PBMCs obtained from healthy donors in vitro, creating conditions specific to psoriasis by activating the cells (e.g., by mitogenic phytohaemagglutinin). They obtained results confirming the inhibition of pro-inflammatory cytokine secretion by the investigational drug. The stimulation of PBMCs by mitogenic phytohaemagglutinin in the presence of IL-12 increased the secretion of IFN-β, an effect inhibited by ustekinumab. IL-23 was found to increase the production of IFN-γ to a much lesser extent compared to IL-12. In vitro studies on 2D cultures, however, are not a reliable reflection of the reactions taking place throughout the body as the pathways of inflammation induction are very complex and their regulation is complicated. In studies on the effect of a drug on gene expression, one should also consider individual variability, which also affects the final results; therefore, it is very important to identify potential markers that supplement treatment monitoring.
Ustekinumab is one of those drugs, the effectiveness of which has been confirmed by numerous trials, mainly based on the dermatological indicators: PASI, DLQI and BSA [25, 30, 31]. This was reflected in our research where were recorded correlations between the PASI index and the BSA index at weeks 16, 28 and 40 of ustekinumab treatment. In our study, we also observed that only IL-8 expression was statistically significantly positively correlated with the BSA index at week 40 of treatment.
Tsai et al. [25] conducted a study on a group of 121 patients suffering from psoriasis and psoriatic arthritis, treating them with ustekinumab. According to their observations, a significant improvement was observed in week 12 of therapy, which correlated with the value of the PASI index, which is consistent with the results described in our study. The PASI result was compatible with the DLQI results, the value of which decreased, which indicates an improvement in the quality of life of patients [25].
In turn, Kavanaugh et al. [30] conducted a two-year study on 615 patients with articular psoriasis, during which they observed the impact and safety of ustekinumab on the course of the disease. Throughout the study period, the ustekinumab group showed a greater reduction in clinical symptoms of the disease, both in joints and skin, and improved quality of life, compared to the placebo group. They also compared their studies with the results of anti-TNF drugs, and thus concluded that ustekinumab exhibits faster efficacy, as measured by the PASI scale, than etanercept. This confirms that ustekinumab therapy is effective in articular psoriasis [30].
Bagel et al. [31] compared PASI and DLQI values between secukinumab and ustekinumab therapy. The values of dermatological indices during treatment with ustekinumab are analogous to those described in our studies. For the first 16 weeks, in both cases, there is a sudden drop in DLQI values, which indicates that the patients’ quality of life has improved significantly. In the described studies on articular psoriasis, the decrease after 16 weeks, however, was slightly lower (on average up to DLQI 5) than in the study of cutaneous psoriasis by Bagel et al. [31] (DLQI 0/1). The PASI values in both cases were similar, which also confirms the thesis about the effectiveness of ustekinumab use [31].
In the study, we also tried to analyse the correlations between the change in IL-6, IL-8, and IFN-γ gene expression and the change in dermatological index values. Unfortunately, at this stage, we are not able to indicate which genes could be superior over others. This may be related to the difference in the rate of phenotypic and molecular changes. In our opinion, expanding the study group with new participants and introducing analyses at the protein level could contribute to drawing such conclusions.
Conclusions
Quantitative assessment of IL-6 and IFN-γ mRNA in PBMCs may be helpful in monitoring ustekinumab therapy. The increase in mRNA copy numbers of the pro-inflammatory IL-6 and IFN-γ genes in the following weeks of therapy may suggest that psoriasis patients may progressively develop resistance to biological treatment.
The results are promising, but they require continuation in a larger group of patients, extension of treatment monitoring for another month, and additional confirmation by proteomic analysis. This will help to identify further molecular markers useful in assessing the effectiveness of ustekinumab therapy in the future.








