Introduction
Shrimp sensitization is an increasing problem in developed countries. Crustacea are a rich source of proteins which may be the cause of hypersensitivity. Several allergenic proteins have been described so far. There are currently many diagnostic possibilities in the case of crustaceous sensitization, including skin prick tests, specific IgE (sIgE) concentration assessment, both with allergen extracts and components. There are also novel, in vitro methods, that are not yet available commercially, such as basophil activation tests that are highly promising and safe in vitro alternatives to Oral Food Challenge (OFC). Despite the progress in the diagnosis of food allergy, the gold standard and the only diagnostic tool that allows discrimination between allergic and non-allergic sensitized patients is OFC, optimally in the form of a double-blind placebo-controlled food challenge [1, 2].
Component-resolved diagnosis is becoming increasingly accessible to patients with different allergy-related problems. Available diagnostic tools include highly specific and sensitive methods, like ImmunoCap Singleplex and microarray tests (ALEX and ImmunoCap ISAC). Each of those diagnostic tools has its benefits and limitations. In this research, we concentrated on the usefulness of the ALEX microarray test in the diagnosis of patients with confirmed shrimp sensitization. We analysed the allergen profile of those patients, including co-sensitization to both food and inhaled allergens.
ALEX2 Allergy Explorer (ALEX®; MacroArray Diagnostics, Vienna, Austria) is an in vitro assay for the measurement of allergen-specific IgE antibodies in human plasma, intended to aid in the diagnosis of IgE-mediated sensitization. It contains 117 allergenic extracts and 178 molecular components, with a vast majority of aeroallergen and food protein families represented. It measures both total and specific IgE against allergen extracts and molecular allergen components [3].
We previously researched the clinical utility of the ImmunoCap ISAC test and ImmunoCap Singleplex in the diagnosis of shrimp allergy. The main limitation of ImmunoCap ISAC, as of any microarray allergen platform, is a limited allergen component list available for diagnosis and lack of allergen extracts in the test. Therefore, in the case of shrimp sensitization, we cannot exclude that the patient is allergic to an allergen component that is absent in the chosen diagnostic tool [4, 5].
The key to correct diagnosis is a qualification to the most efficient diagnostic method and later interpretation of the result. To achieve this, it is necessary to apply a diagnostic strategy relevant to each patient’s situation and approach every case individually. Due to the relatively high cost of component resolved diagnosis, it is crucial to choose an immunological method that will give both the physician and the patient the most precise and complete information.
Aim
In this study the allergen profile of shrimp-sensitized patients was analysed using ALEX2 Allergy Explorer.
Material and methods
This study includes 50 adult patients (28 women and 22 men), who were the patients of the Allergology Ward or Outpatient of the Clinic of Allergology, Clinical Immunology and Internal Medicine in Bydgoszcz, Collegium Medicum Nicolaus Copernicus University (NCU), because of suspected food allergies.
The main eligibility criterion for the study was the presence of a positive prick-by-prick test with tiger shrimp bought from the local eco-market and an elevated concentration of IgE specific to shrimp allergen extract (ImmunoCap).
A total of 35 patients (26 women and 9 men) with negative skin prick tests with shrimp and not detectable sIgE shrimp in ImmunoCap were included in the control group.
Patients being treated for serious chronic diseases, as well as those on medication (e.g., antihistamines, systemic steroids, immunotherapy, and β-blockers) that could influence the results of this study were excluded.
A detailed history of allergies was taken and a physical examination was conducted for each patient. All the patients had skin prick tests with food allergens using the Allergopharma set (wheat flour, egg, milk, peanuts, fish, walnuts, soy, celery, apple, and shrimp) as well as prick-by-prick tests with tiger shrimp bought from a local eco-market. As per the current European standard, test results were considered positive when the diameter of the wheal of each particular test was ≥ 3 mm.
The immunological assay in the study was conducted using venous blood serum. Blood was taken following the standard conditions, between 7:00 am and 9:00 am. Patients were under overnight fasting. Blood was collected from the median cubital vein using a closed vacuum system (Vacuette, Greiner Bio-One), into ‘CAT Serum Sep Clot Activator’ 5 ml tubes. Once the clots are fully formed in the blood samples, they were centrifuged for 15 min at 3,500 rpm. The serum was immediately separated and frozen at –70°C until the assay.
Shrimp-specific IgE (Pandalus borealis, Penaeus monodon, Metapenaeopsis barbata, and Metapenaeus joyner extract) immunological determinations were performed using the highly sensitive immune-fluorescent ImmunoCap method (Thermo Fisher Scientific). Concentrations of sIgE were found to increase when they exceeded 0.35 kUA/l (ImmunoCap), following the common practice in the field.
ALEX2 Allergy Explorer was developed by MacroArrayDX (Vienna, Austria). The test is commercially available, having attained CE certification, which assures the quality of the assay. All the different allergens and components are spotted onto a nitrocellulose membrane in a cartridge chip, which is then incubated with 0.5 ml of a 1 : 5 dilution of serum under agitation. The unique aspect of this microarray test is the serum diluent contains a CCD inhibitor. After incubation for 2 h, the chips are extensively washed, and a pre-titered dilution of anti-human IgE labelled with alkaline phosphatase is added and incubated for 30 min. Following another cycle of extensive washing, the enzyme-substrate is added, and after a few minutes, the reaction is complete. The membranes are dried, and the intensity of the colour reaction for each allergen spot is measured by a CCD camera. The dedicated software digitalizes the images and prepares a report that lists the allergens and components and their score in kUA/l [6].
In ALEX 2 there are several allergen extracts and allergen components related to shrimp. The most important allergen extracts include those of shrimp mix (Litopenaeus setiferus, Farfantepenaeus aztecus, Farfantepenaeus dourarum), Northern prawn (Pandalus borealis), and allergen components of Black-Tiger shrimp (Penaeus monodon): Pen m 1 (Tropomyosin), Pen m 2 (Arginine Kinase), Pen m 3 (Myosin light chain) and Pen m 4 (Sarcoplasmic Calcium Binding Protein). What is more, there is one allergen component of Brown shrimp (Crangon crangon) – Cra c 6 (Troponin C).
Apart from those there are also several allergen components and extracts not directly related to shrimp, but with potential cross-reactivity. Those would include house dust mite allergens: European house dust mite (Dermatophagoides pteronyssinus) – rDer p 1 (Cysteine Protease), rDer p 2 (NPC2 Family), rDer p 5, rDer p 7 (Mite Group 7), rDer p 10 (Tropomyosin) rDer p 11 (Myosin, heavy chain), rDer p 20 (Arginine Kinase), rDer p 21, rDer p 23 (Peritrophin-like protein domain). Also, potent allergens are from other types of seafood, such as allergen extracts of common mussel (Mytilus edulis), Crab (Chionoecetes spp.), Lobster (Homarus gammarus), Oyster (Ostrea edulis), Squid (Loligo spp.), Venus clam (Ruditapes spp.) or allergen components of Anisakis simplex rAni s 1 (Kunitz Serine Protease Inhibitor), and rAni s 3 (Tropomyosin).
The concentration of those allergen extracts and components of interest were analysed to establish the sensitization pattern in the group of shrimp-sensitized patients.
Results
The study group consisted of 50 adult patients (28 women and 22 men), aged 19–75 (mean 42), and the control group of 35 patients (26 women and 9 men), aged 19–75 (mean 43.7). The characteristics of the study and control group are presented in Table 1.
Table 1
The characteristics of the study and control groups
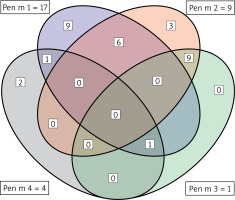
The results of the ALEX2 test were analysed. Out of the group of 50 patients sensitized to the shrimp allergen extract in ImmunoCap, 22 were sensitized to at least one allergen component of Penaeus monodon. The number of patients sensitized to each of the allergen components is presented in Figure 1.
Figure 1
Shrimp sensitisation in the research group – number of patients sensitized to shrimp allergen components in ALEX2. Venn graph
The concentrations of shrimp and seafood allergen extracts are presented in Table 2.
Table 2
The concentration of shrimp and seafood allergen extracts in the research and control groups
There were no patients with elevated concentrations of shrimp or seafood allergen extracts in the control group. In the shrimp-sensitized group, 20 patients were sensitized to crab, and 20 were sensitized to lobster. What is surprising, only 15 (30%) patients were sensitized to the Northern prawn (Pandalus borealis) allergen extract in ALEX2 and only 12 (24%) to Shrimp mix (Litopenaeus setiferus, Farfantepenaeus aztecus, Farfantepenaeus dourarum). Interestingly, 27 (54%) patients were sensitized to at least one allergen extract of shrimp or seafood, and 3 patients were sensitized to all nine analysed allergen extracts, 4 patients to eight allergen extracts, and 18 in total were sensitized to more than one allergen extract.
Another interesting aspect of the study was potential cross-sensitization between the concentration of shrimp tropomyosin Pen m 1 and Anisakis simplex tropomyosin Ani s 3. The results of the analysis are presented in Figure 2.
Figure 2
The concentration of sIgE Pen m 1 and sIgE Ani s 3 in patients sensitized to shrimp based on ALEX2 microarray test. Values of sIgE concentration for specific patients are presented in the lower part of the figure
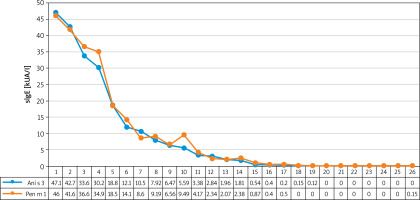
The correlation between the concentration of sIgE Pen m 1 and sIgE Ani s 3 in patients sensitized to shrimp is high – 0.98 (Spearman’s Rank Correlation).
A similar result was achieved for correlation between sIgE Pen m 1 and sIgE Per a 7, an American cockroach (Periplaneta americana) tropomyosin – 0.98 (Spearman’s rank correlation).
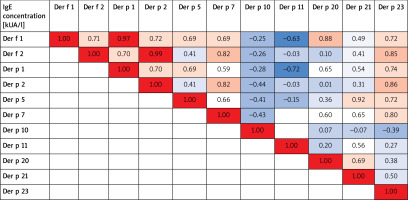
The correlation between the concentration of sIgE Pen m 1 and sIgE to house dust mite tropomyosin Der p 10 in patients sensitized to shrimp was moderate to high – 0.75 (Spearman’s rank correlation).
In the shrimp-sensitized group, co-sensitization to house dust mite allergen components was common and related to a wide range of protein families – Table 3. As many as 41 (82%) patients were sensitized to at least one allergen component available in ALEX 2.
Table 3
House dust mite sensitization profile of shrimp-sensitized patients based on ALEX2 microarray test
Sensitization to HDM Der p 2, Der p 23, Der p 5, and Der p 1 was the most common (66%; 54%; 52%, and 52%, respectively). The rate of HDM sensitization was significantly higher in the shrimp sensitization group, compared to the control group (p < 0.05).
A vast majority of patients were sensitized to more than one Dermatophagoides pteronyssinus allergen component or Dermatophagoides farinae allergen components. The Spearman’s rank correlation between the concentration of HDM allergen components in the research group is presented in Figure 3. An especially strong correlation was noted between sIgE HDM allergen components groups 1 and 2 from both analysed HDM species (0.97 and 0.99, respectively). A strong correlation was also observed between the concentration of sIgE Dep p 5 and sIgE Der p 20 (0.92).
Discussion
There are several important practical aspects of the study. The whole research group consisted of patients with elevated concentrations (≥ 0.52 kUA/l) of IgE specific to the shrimp allergen extract (Pandalus borealis, Penaeus monodon, Metapenaeopsis barbata, and Metapenaeus joyner extract) in the highly specific and sensitive method – ImmunoCap Singleplex. ALEX 2 test allows to access both allergen extracts and allergen components. There are two shrimp allergen extracts available in this microarray test – Northern prawn (Pandalus borealis) and Shrimp mix (Litopenaeus setiferus, Farfantepenaeus aztecus, Farfantepenaeus dourarum). The sensitivity of ALEX2 was relatively low with only 15 (30%) patients and 12 (24%) patients with elevated sIgE, respectively. One of the possible explanations is due to the difference in shrimp species assessed using the Singleplex ImmunoCap and Allergy Explorer-ALEX2 method. But it may also be related to the relatively low sensitivity of ALEX2 in general in shrimp allergy, at least in the case of allergen extracts.
Buzzulini et al. published interesting research work in 2019. The authors compared results of skin prick tests, ImmunoCap and Allergy Explorer-ALEX in 105 patients and 15 negative controls and found a substantial agreement between ALEX and ImmunoCap as shown on the detection of IgE to extracts, notably lower for inhalants than food allergens (k = 0.64 and k = 0.51, respectively). What is interesting, there was a higher agreement on detection of molecular components (k = 0.92 for inhalants and k = 0.72 for food allergens) than allergen extracts [7]. Those results are in line with our findings.
In our research, 17 (34%) patients were sensitized to shrimp tropomyosin, Pen m 1. It is considered a major shrimp allergen, responsible for interspecies cross-reactivity between various other crustaceans and arachnids, such as crayfish, lobsters, crabs, and house dust mites. It is heat stable and may cause anaphylaxis [8, 9]. In our previous research on ImmunoCap ISAC in shrimp sensitization in different shrimp-sensitized populations, tropomyosin was detected in 28% of the patients, which is a similar result although achieved using different immunological methods [10].
The relatively low prevalence of tropomyosin sensitization in the current study population was consistent with the results of other studies. One might suspect that there are other allergen components responsible for sensitization in patients with low detectable levels of IgE specific to Pen m 1, Pen m 2, Pen m 3, and Pen m 4. Asero et al. found that 41% of patients who were shrimp sensitized had elevated levels of sIgE against tropomyosin. A particularly interesting aspect is that 52% of patients were sensitized to a protein of molecular weight > 60 kDa. Allergy to other shrimp allergen components – arginine kinase (Pen m 2, 40 kDa), calcium-binding sarcoplasm binding protein (Lit v 4, 20 kDa), light myosin chain (Lit v 3, 20 kDa), triphosphate isomerase (Cra c 8, 27 kDa), troponin C (Cra c 6, 17 kDa), and fatty acid-binding protein (15 kDa) was rarely observed (13% of patients) [11]. Later Giuffrida et al. identified the high molecular weight protein (> 60 kDa) as hemocyanin [12]. Although hemocyanin was first identified in 2014, today there are still no commercially available methods of determining levels of sIgE against this allergen. It can be suspected as a source of an allergic reaction, up to anaphylaxis, in patients not sensitized to available shrimp allergens. A striking aspect of hemocyanins is that they are considered to be among the allergens responsible for cross-reactivity of HDM allergens [13]. In the present study, moderate to high Spearman’s Rank Correlation was found between tropomyosin from different origins – Anisakis simplex, American cockroach (Periplaneta American), and house dust mite (Dermatophagoides pteronyssinus). The problem of cross-reactivity in the case of tropomyosin is widely recognized [14]. Homology between amino acid sequences reported in allergen databases of selected invertebrate tropomyosin was determined with Der f 10 as the reference allergen. The 66.9 and 54.4% identities were found with selected crustacean and insect species, respectively, whereas only 20.4% identity was seen with molluscs [15]. Reese et al. studied sequence homology of M. ensis (Met e1) and Pen i1 with allergens from other sources and found 98% homology in both the allergens with Hom a1 (Atlantic lobster), 98% with Pan s1 (spiny lobster), 82% Per a7 (American cockroach), and 81% with Der p10 (HDM) [16]. In a new study by Laurchan et al., tropomyosin allergenicity in four freshwater crustacean species: prawn (Macrobrachium rosenbergii and Macrobrachium lanchesteri), and crayfish (Procambarus clarkii and Cherax quadricarinatus) were analyzed. Cloning and characterization of nucleotide sequences of tropomyosin cDNA from M. lanchesteri and C. quadricarinatus revealed highly conserved amino acid sequences with other crustaceans [17].
The second most prevalent shrimp allergen component in the group was arginine kinase, which sensitized 9 (18%) patients. This protein is found only in invertebrates, in the vertebrates, its counterpart is creatinine kinase. Arginine kinase is involved in ATP transformations and phosphoarginine synthesis [18]. Studies conducted in Italy have shown that it is a minor allergen that sensitizes 10–15% of the population of patients allergic to shrimp allergens, which is consistent with our findings [12].
Only 1 patient was sensitized to myosin light chain 2, Pen m 3. This Penaeus monodon is recognized since 2019 and is available commercially only in one diagnostic method – ALEX2, and is not available in the previous version of ALEX, and in other diagnostic methods, like ImmunoCap ISAC, Faber Multiplex platform, or ImmunoCap Singleplex [19, 20]. In general, myosin light chain from other shrimp species, Litopenaeus vannamei, was described in 2008 by Ayuso et al. The authors demonstrated the presence of light-chain-specific myosin IgE in 31 of the 38 analyzed sera of shrimp-allergic patients (55%). Significant homology of the sequence of Lit v 3 of the shrimp with cockroach Bla g 8 has also been demonstrated [21].
Four patients were sensitized to Pen m 4, Sarcoplasmic calcium-binding protein, muscle protein, which plays an important role in the process of muscle contraction. Interesting work was published by Wang et al. in 2012. Sequential homology of 81–100% has been demonstrated between sarcoplasmic calcium-binding protein found in crustaceans of various species (Metapenaeus ensis, Penaeus monodon, Oratosquilla oratoria, Macrobrachium rosenbergii, Procambarus clarkii, Portunus pelagicus, Charybdis feriatus, Eriocheir sinensis). Like arginine kinase, it is a smaller allergen of clinical significance, sensitizing about 10–15% of patients who have been shown to have allergen-specific shrimp IgE [12, 21].
Another interesting aspect of the study is the fact that 40% of the shrimp-sensitized group had an elevated concentration of IgE specific to crab (Chionoecetes spp.) and lobster (Homarus gammarus). In the control group, none of the patients shared this sensitization. In the group, many patients were sensitized to squid (Loligo spp.), common mussel (Mytilus edulis), oyster (Ostrea edulis), scallop (Pecten spp.), and venus clam (Ruditapes spp.) – Table 2. There are many cross-reacting protein, that can explain the fact that the shrimp allergy coexists with an allergy to another type of seafood. These mainly include tropomyosin, but also arginine kinase [22, 23]. There was a unique case report published in 2009 on selective allergy to lobster in a case of primary sensitization to house dust mites. The 30-year-old patient’s serum recognized 2 allergens of around 198 kDa and 2 allergens of around 65 kDa from the lobster extract, allergens of around 15, 90, and 120 kDa from the Dermatophagoides pteronyssinus extract, and allergens of around 15 and 65 kDa from the Dermatophagoides farinae extract. The serum did not recognize purified shrimp tropomyosin. Immunoblot-inhibition assay results indicated cross-reactivity between lobster and mite allergens [24].
In the current research, it was confirmed that HDM sensitization often occurs in shrimp-sensitized patients. At least 82% of shrimp-sensitized patients had elevated concentrations of sIgE to at least one HDM allergen component available in ALEX 2. In the control group, only 31.4% of patients were sensitized to at least one HDM allergen component. The allergen components of house dust mites are relatively well described [1]. In our population, a vast number of patients were sensitized simultaneously to HDM allergens from different protein families. The most common in the research group was sensitization to Der p 2 (66% of patients), Der p 23 (54%), Der p 1 (52%), and Der p 5 (52%).
Cysteine protease (Der p 1, Der f 1), an allergen present in mite droppings, together with an allergy to group 2 mite allergens, is the predominant cause of clinical symptoms in patients allergic to house dust mites. It is worth emphasizing that cysteine protease mites in contact with the human mucous membrane are an active enzyme that increases the permeability of the allergen, which further aggravates the symptoms. Studies show that Der p 1 and Der f 1 have a homologous sequence of 81%. Trombone et al. showed that 95% of patients whose D. pteronyssinus – specific IgE levels are above 2 kU/l have elevated Der p 1 or Der p 2-specific IgE [25, 26]. NPC2 (Der p 2, Der f 2) is an allergen present in mite droppings, interesting in terms of molecular structure as it consists of protein fragments arranged around a space with the ability to bind lipids. Der f 2 has been shown to bind liposaccharides [25]. An interesting work was published in 2016 by Sylvestre et al. The authors analyzed the results of the ImmunoCap ISAC study conducted in 126 patients with atopic bronchial asthma. They showed that in patients with severe asthma, allergy to the allergen components of mites available in ImmunoCap ISAC was more common than in patients with moderate and mild bronchial asthma. This relationship was particularly strongly expressed in the case of allergy to Der p 2 and Der f 2 [27]. Group 5 mite allergens (Der p 5, Blo t 5) is a protein resistant to high temperature, secreted by the cells of the epithelium of the middle intestine of mites. Der p 5 has been shown to sensitize 31% of patients allergic to dust mites. No significant cross-reaction between IgE Der p 5 and Blo t 5 has been demonstrated [28]. Der p 23 is a peritrophin-like protein, the main, inhaled allergen, which is an IgE-binding protein, usually in low titre. It is found in the excrement of mites [29]. Previous studies have demonstrated that Der p 23 elicits a positive sensitization rate of 75% in patients with house dust mite (HDM) allergies, particularly in children with persistent moderate-to-severe asthma [30]. Martín-López in 2021 published the first report on environmental exposure to Der p 23. Twenty-nine dust samples were collected in Lanzarote (Canary Islands, Spain). Der p 23 was detected in all samples except for two, in which the levels were below the limit of detection. The mean level of Der p 23 was 1.85 ±2.62 µg/g of dust (range: 0.3–11.45 µg/g of dust), which was approximately four-fold lower than the mean levels of Der p 1 and Der p 2 (7.74 ±9.36 and 7.35 ±10.36 µg/g of dust, respectively) [31].
The allergen components of house dust mite are most commonly associated with cross-sensitization with shrimp in tropomyosin. Diez et al. in 2021 published interesting research on 443 patients with allergic rhinitis. Eighty-six (19.4%) patients were sensitized to shrimp and 23 of them (26.7%) had shrimp allergy diagnoses. Thirty-six of the patients sensitized to shrimp (41.2%) reported that they did not previously consume them, but eleven of them had a positive oral challenge test (30.5%). Anti-Der p 10 IgE was associated with a risk of a positive oral food challenge with shrimp [32].
In research by Boquete et al., it was found that 71% of the patients allergic to HDMs also had IgE specific to shrimp and 55% of them had increased levels of IgE specific to shrimp tropomyosin [33]. In our research, sensitization to HDM tropomyosin was present in 32% of shrimp-sensitized patients, and shrimp tropomyosin Pen m 1 in 34% of shrimp-sensitized patients. On the other hand, Canadian studies demonstrated a high incidence of allergy to HDMs in 95 patients with a confirmed allergy to shrimp. In that study population, 86 (90.5%) patients had positive skin prick tests for HDM allergens [34], which would be in line with our findings – 82% of shrimp-sensitized population had simultaneously elevated HDM-specific IgE.
The limitations of this study are a relatively small population and lack of oral food challenge to confirm shrimp allergy symptoms. Using ALEX2 as a main diagnostic tool gives a lot of data to analyse, but may lack sensitivity, especially in the case of allergen extracts. Using a wider range of ImmunoCap Singleplex might give better insight into the specificity and sensitivity of this microarray diagnostic method. Further research, which would include double-blind placebo-controlled food challenge and more patients, would be required to assess the concentration of sIgE characteristics to symptomatic patients.
Conclusions
The simultaneous detection of both extracts and molecular components with the ALEX2 assay can potentially overcome some of the major limitations of the multiplex assay. On the other hand, ImmunoCap Singleplex remains the most reliable in vitro diagnostic tool to assess sIgE, with the highest available specificity and sensitivity. Our research confirms that in the case of shrimp allergen extracts it is better to rely on ImmunoCap Singleplex than ALEX2, due to the lower sensitivity of the microarray test.
Sensitization to house dust mites is an important problem in shrimp-sensitized patients. What is more, shrimp sensitization was confirmed to coexist with sensitization to other types of seafood, probably due to cross-reactivity with several important allergens.
Sensitization to shrimp tropomyosin in the research group was present only in 34% of cases. There may be other shrimp allergen components, not available in ALEX2, which are responsible for shrimp sensitization.
ALEX2 gives a wide insight into the allergen profile of patients, although due to a large number of extracts and components analyzed, requires a careful interpretation combined with a wide and precise allergic interview and specific clinical situation of each patient.