Introduction
Scleroderma is a chronic connective tissue disease of unknown aetiology. Concomitant immunological disorders with lesions of an inflammatory and subsequently vascular nature are suggested in this respect. Progressive fibrosis is considered as the final stage in the pathogenetic chain of the disease. The condition is not classified as genetically conditioned, but hereditary factors may actually be involved in its development. Qualitative disturbances of the connective tissue and collagen metabolism are not observed. One distinguishes two main clinical forms of the disease: systemic scleroderma (which has two subtypes: the localized form and the generalized form) and localized scleroderma (also referred to as morphea and LoS) [1]. LoS lesions are usually confined to the skin and the subcutaneous tissue, which distinguishes localized scleroderma from systemic scleroderma as the fibrosis process in the latter also affects internal organs. Nonetheless, the diseases have many common features such as dominant activity of Th2 lymphocytes during an inflammatory reaction, endothelial cell dysfunction and a similar histopathological picture. Many authors reckon that those two disease entities are closely related, but should be investigated separately [2–8].
The available publications present results of numerous studies concerning increased concentrations of various particles related to the pathogenesis of localized scleroderma in the blood serum of patients with LoS in comparison with control groups as well as their relation to the clinical form of the disease, the number of skin lesions, the number of body areas affected by the disease and disease duration [1, 3, 8–11]. However, only a few studies take into account all those parameters simultaneously.
One of the cytokines with a significant role in the pathogenesis of numerous inflammatory and autoimmune diseases is tumour necrosis factor-α (TNF-α). Binding TNF- a to membrane-bound receptors creates soluble forms: sTNFαR1 and sTNFαR2. Studies highlight that concentrations of sTNFαR1 and sTNFαR2 in the blood serum of patients with localized scleroderma correlate with their in situ expression in tissues as well as with laboratory and clinical indicators of the inflammatory condition and disease progress [9–16]. The significance of sTNFαR1 as a parameter determining activity, severity and therapy effectiveness for localized scleroderma has not been studied yet.
This study aims to assess the concentration of the cytokine related to one of the most important sclerodermic lesion development factors – immune system activation in patients with localized scleroderma, as well as analyse the connection between sTNFαR1 and the clinical picture of the disease. The work investigates the correlation of sTNFαR1 concentration in the cases where antinuclear antibodies are present in the blood serum as well as compares receptor concentrations before and after the therapy depending on the treatment method and disease duration. The study attempts to determine whether sTNFαR1 concentration may be a prognostic marker for the coexistence of localized scleroderma and the systemic form.
Aim
The main aim is an assessment of sTNFαR1 concentration in the blood serum of patients with localized scleroderma in comparison with a control group composed of healthy persons. The second goal of the study is determination of relationships between sTNFαR1 receptor plasma concentration and disease duration, scleroderma form, the presence of antinuclear antibodies as well as the skin severity index and the skin damage index, and also assessment of sTNFαR1 plasma concentration before and after treatment depending on the therapeutic method.
Material and methods
The study covered 41 patients with localized scleroderma: 35 women and six men aged 19–68 (mean ± SD: 48.8 ±14.6). The control group comprised 20 healthy persons: 12 women and eight men aged 19–68 (mean ± SD: 47.10 ±14.66). The patients with localized scleroderma in the studied group were treated using two methods: photochemotherapy or an antibiotic therapy (20 patients – PUVA therapy, 21 patients – PNC). Topical treatment was limited to magistral greasing preparations and emollients throughout the observation period. In the case of the patients treated with intramuscularly administered procaine penicillin (dose: 2.4 million IU/day), achievement of a total dose of at least 30 million IU/day was considered as the end of the therapy. In the group of patients treated with photochemotherapy, the single initial dose during a PUVA session was 0.5 J/cm2 and it was increased by 0.5 J/cm2 every other day to reach the maximum value of 10 J/cm2, depending on the clinical condition. The study involved three sessions a week. The session scheme, determined based on the literature data, was subsequently modified depending on the patient’s individual response during the treatment. It was decided that the observation would end after 20 sessions. The patients took methoxsalen an hour before the session; its dose depended on the body weight (up to 40 kg – 10 mg; 40–50 kg – 20 mg; 50–60 kg – 30 mg; more than 60 kg – 40 mg).
The study investigated patients for whom disqualifying factors were excluded and who demonstrated clinical symptoms characteristic for localized scleroderma and confirmed by a histopathological examination.
All the study participants underwent:
determination of sTNFαR1 blood serum concentration using the ELISA (enzyme-linked immunosorbent assay) method,
capillaroscopy.
In the patients with LoS, the following were additionally assessed:
clinical form of the disease (plaque, linear or generalized),
location of skin lesions (disease foci present in the upper or lower part of the body or in both those regions),
disease activity duration (from the appearance of the first signs/symptoms for new cases or from the moment of reactivation of previously existing LoS foci – extension of lesions, appearance of erythema or intensification of skin hardening),
skin severity index (based on mLoSSI – modified Localized Scleroderma Skin Severity Index [17]; 18 separate body areas were assessed in terms of the presence of new or extension of old lesions as well as intensification of erythema and skin hardening),
skin damage index (based on LoSDI – Localized Scleroderma Skin Damage Index [18]; skin damage was assessed analogously to the mLoSSI scale – score: 0–3). The following features were assessed in 18 anatomical areas of the body: atrophy of skin and subcutaneous tissue as well as discolorations.
Results
Concentration analysis of the determined particle in the studied group of patients with localized scleroderma and in the control group of healthy persons
Statistically significantly higher sTNFαR1 concentrations were observed in the studied group of patients with localized scleroderma in comparison with the control group (p < 0.005). Their range was 78.30–1326.50 ng/ml (median: 543.50 ng/ml) in the patients and 159.80–349.65 ng/ml (median: 254.60 ng/ml) in the control group (Table 1).
Table 1
Comparison of studied particle concentrations in the group of patients with localized scleroderma and in the control group
A statistically significant difference was established between the studied group and the control group (p < 0.005). sTNFαR1 values were higher in the studied group than in the control group (Table 2).
sTNFαR1 concentration analysis in the studied group of patients with localized scleroderma depending on selected clinical parameters
The patients participating in the study (n = 41) were divided into 3 subgroups depending on the clinical form of the disease. 19 persons were diagnosed with plaque scleroderma, 16 – with generalized scleroderma and 6 – with linear scleroderma. An analysis of sTNFαR1 concentrations depending on the LoS subtype showed no statistically significant differences.
An analysis of sTNFαR1 concentrations before and after treatment in the patients with localized scleroderma depending on the skin severity index (mLoSSI) revealed no statistically significant changes.
The studied group demonstrated a statistically significant positive curvilinear relationship between the skin damage index and sTNFαR1 concentration tested after treatment completion. Patients with a higher skin damage index also had a higher blood serum concentration of the determined parameter. It was a weak correlation (p = 0.33) (Table 3, Figure 1).
Table 3
Coefficients of correlation between mLoSSI and mLoSDI and sTNFαR1 after treatment
| mLoSSI after treatment | mLoSDI after treatment | |
|---|---|---|
| sTNFαR1 after treatment | 0.18 | 0.33 |
Figure 1
Scatterplot for mLoSSI and mLoSDI after treatment (B) expressed in points and sTNFαR1 after treatment (B)
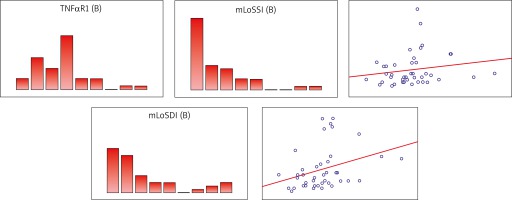
An analysis of sTNFαR1 concentration depending on the disease process activity time in the studied group of patients with LoS showed no relationship between those parameters.
Analysis of the relationship between sTNFαR1 blood serum concentration before and after treatment and effectiveness of a given therapeutic method
The patients from the studied group were divided into two subgroups depending on the treatment method. The subgroup treated with procaine penicillin (PNC) included 21 patients and the subgroup undergoing PUVA therapy comprised 20 patients. sTNFαR1 blood serum concentration was determined in all the study subjects before and after treatment. The TNF aR1 level dropped after therapy completion in both groups, regardless of the treatment method. The difference in the determined particle level was higher in the group of patients undergoing photochemotherapy (median: 106.25 ng/ml) than in the group taking penicillin (median: 81.50 ng/ml) (Tables 4 and 5).
Table 4
sTNFαR1 concentration difference before and after treatment depending on the treatment method
Analysis results of the skin severity index and the skin damage index depending on selected clinical parameters
Analysis of the difference between mLoSSI and mLoSDI/LoSDI results before and after treatment depending on the therapeutic method.
Mann-Whitney U test was conducted to search for any statistically significant differences between mLoSSI and mLoSDI results before and after treatment depending on the therapeutic method. Before the analysis, the mLoSSI score before the treatment was corrected by taking into account the presence of new foci. The analysis allows one to conclude that there are statistically significant differences in results depending on the treatment method (p < 0.05) (Table 6, Figures 2, 3).
Table 6
Results of Mann-Whitney U test of independence of variables depending on the treatment method
| Average rank PNC | Average rank PUVA | Z | P-value | Number of patients treated with PNC | Number of patients treated with PUVA | |
|---|---|---|---|---|---|---|
| mLoSSI | 14.4 | 28.0 | –3.66 | 0.000 | 21 | 20 |
| mLoSDI | 14.2 | 28.2 | –3.87 | 0.000 | 21 | 20 |
Figure 2
Order statistics for mLoSSI score differences before and after treatment depending on the treatment method
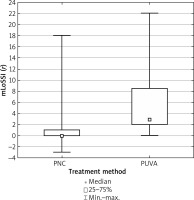
Figure 3
Order statistics for mLoSDI score differences before and after treatment depending on the treatment method
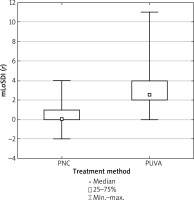
The χ2 test, conducted to determine the possible improvement after the treatment, also showed differences between the two therapies. A higher improvement rate was obtained for PUVA. Nineteen out of 20 study subjects undergoing PUVA therapy demonstrated a decrease in the skin severity index. A skin damage index decrease was observed in 17 out of 20 patients treated using that method. Most patients taking procaine penicillin showed no improvement after treatment completion. Both the skin severity index (mLoSSI) and the skin damage index (mLoSDI) show a moderate relationship between the improvement of results and the treatment method (Tables 7 and 8).
Table 7
Disease severity change before and after treatment assessed using the mLoSSI scale depending on the treatment method.
Table 8
Skin damage index change before and after treatment assessed using the mLoSDI scale depending on the treatment method
Influence of the clinical subtype of localized scleroderma on the parameter analysis results
Antinuclear antibodies (ANA) were most commonly positive in the patients with the generalized subtype of scleroderma. Approximately a half of the patients with that disease form showed a positive result. The titre of those antibodies was also positive in 31.3% of patients with the plaque form, while the patients with the linear subtype had no ANA at all (Table 9).
Table 9
The presence of ANA depending on the clinical subtype of the disease
| LoS form | ANA | ||
|---|---|---|---|
| (–) Negative | (+) Positive | Total | |
| Plaque | 11 (68.8%) | 5 (31.3%) | 16 |
| Generalized | 10 (52.6%) | 9 (47.4%) | 19 |
| Linear | 6 (100.0%) | 0 (0.0%) | 6 |
| Total | 27 | 14 | |
| χ2 | 4.648 | ||
| P-value | 0.098 | ||
| V | 0.337 | ||
No relationship was observed between the clinical subtype of the disease and the presence of a positive titre of Scl-70, ACA and RNA antibodies.
Discussion
Many cytokines may participate in the pathogenesis of localized scleroderma by stimulating the synthesis of other pro-inflammatory particles, reducing the activity of extracellular matrix metalloproteinases, stimulating the proliferation of fibroblasts and inducing the expression of adhesive particles on endothelial cells. Numerous publications on soluble tumour necrosis factor-α (TNF-α) receptor type I point to the enormous role of that cytokine in the pathogenesis of connective tissue diseases, but that role has not been investigated yet in patients with localized scleroderma. In systemic scleroderma (SSc), the highest concentrations of sTNFαR1 have been observed in patients with a fast progression of organ lesions. Moreover, it has been established that sTNFαR1 concentration in the initial phase of disease development positively correlates with the Scleroderma Skin Severity Index (skin cores). Higher sTNFαR1 values in SSc patients with a shorter duration of Raynaud’s phenomenon confirm the value of that clinical parameter as a prognostic factor of faster disease progression. The obtained results point to the possibility of using sTNFαR1 concentration determination for assessing the activity and severity of systemic scleroderma [9, 10], but this has not been investigated for the skin form thus far.
The range of publications on morphea is much smaller. Based on the undeniable similarity of the molecular mechanisms leading to excessive fibrosis in both those disease entities and on the available literature, the described study assessed the significance of the TNF a–sTNFαR1 signalling pathway in the course of localized scleroderma.
An assessment of sTNFαR1 concentration in patients treated with two different methods allowed one to compare those strategies and might allow one to make an individual choice of the best therapy in the future depending on the correlation between the studied parameters. The obtained results provide new information on the potential mechanisms leading to localized scleroderma and make the hypothesis of a close relationship between that disease and the systemic form of scleroderma less probable.
The presented own study revealed increased sTNFαR1 concentrations in the patients with localized scleroderma in comparison with a control group of healthy volunteers. The analysis showed no correlation between the concentration of the studied cytokine and the skin severity index in localized scleroderma. This points to the role of sTNFαR1 in the pathogenesis of the disease.
The significant role of sTNFαR1 as an immune system activity marker in scleroderma was confirmed both in the previous publications and own research. The conducted analysis proved the correlation between sTNFαR1 concentration and the skin damage index. Therefore, it seems a valuable prognostic factor, useful in anticipating the further course of the disease. No relationship between sTNFαR1 concentration and disease duration or clinical subtype of scleroderma was determined.
In more severe cases, Braun-Falco recommends intramuscular administration of procaine penicillin (dosage: 2,400,000 units a day) until a total dose of approx. 30 million units is reached [6]. Based on the results obtained in the own study, PUVA photochemotherapy seems to be a better therapeutic strategy compared with procaine penicillin. Patients treated with PUVA sessions demonstrated a greater decrease in sTNFαR1 concentration and an improvement of the clinical condition after therapy completion. PUVA therapy is a recognized method of LoS treatment as confirmed in numerous scientific publications [19–21].
One has to state that LoS and SSc are entirely different disease entities, but they may share certain pathophysiological and genetic pathways [22]. The probability of localized scleroderma transformation into the systemic form of the disease is slight. According to various sources, that risk equals 0.9 to 5.7% [5, 23]. In a big randomized study, the presence of typical morphea foci was determined only in 12 (3.2%) out of 370 patients with systemic scleroderma (mainly the IcSSc form). It was also established that generalized localized scleroderma was developed earlier than SSc or simultaneously with SSc. Overlapping of several non-skin diseases in the picture of localized scleroderma is always a threat of disease progression. However, internal organs are usually affected less severely in morphea than in the systemic form. Patients with localized scleroderma often require specialized dermatological, orthopaedic, ophthalmological, neurological and rheumatological care as well as intense physiotherapy.
Conclusions
sTNFαR1 concentration in the blood serum of patients with localized scleroderma is statistically significantly higher in comparison with the control group. This points to the role of sTNFαR1 in the pathogenesis of the disease. sTNFαR1 concentration correlates with the skin damage index and therefore is a valuable prognostic factor, useful in anticipating the further course of the disease. No relationship between sTNFαR1 concentration and disease duration, clinical subtype of scleroderma or the skin severity index was determined. Patients treated with PUVA demonstrate greater drops of sTNFαR1 concentration in plasma after therapy completion in comparison with the group treated with PNC. Generalized LoS demonstrated the highest skin severity index and a positive titre of antinuclear antibodies (ANA).








