Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin disease with severe itching. It occurs mainly in the paediatric population, with a frequency of up to ~20% in this group of patients [1, 2]. More and more cases are observed in adults, most often as a continuation of childhood illness. Recently, emphasis has been placed on late-onset atopic dermatitis in the elderly, especially those over 60 years of age, which requires differentiation from other inflammatory skin diseases [3, 4]. The incidence has been increasing steadily for several decades, not only in countries with a higher degree of urbanisation and economy [5]. The number of people suffering from AD is relatively high. The disorders have clear negative social and economic impacts, and treatment remains challenging. Genetic, immunological and environmental factors, disorders of the epidermal barrier function and microbiome play a role in the pathogenesis of the disease [6]. While observing patients diagnosed with atopic eczema, attention was drawn to its coexistence with other inflammatory disorders. Most often other manifestations of allergy and atopy coexist with AD such as asthma, food allergy and allergic rhinitis. Due to the noticeable, similar sequence of diseases, the phenomenon was called allergic march (also known as an atopic triad) [7]. Recently, the relationships and coexistence with other pathologies have also been studied. They suggest a positive correlation between AD severity and the prevalence of these diseases and the relationship between AD and comorbidities is probably bidirectional and multifactorial [8, 9]. Atopic dermatitis is also associated with several mental disorders, especially anxiety and depression [10, 11]. Atopic dermatitis may also be related to infectious diseases, autoimmune disorders, alopecia areata and obesity [9]. Data on malignancy development are controversial, but it has been suggested that severe, long-term atopic dermatitis is associated with adult lymphomas. Particular attention is dedicated to the coexistence of AD with diseases of the cardiovascular system (Figure 1). Patients with AD have many probable risk factors for cardiological diseases, including chronic sleep disorders, sedentary lifestyles, higher rates of smoking and alcohol consumption, and side effects of some systemic therapies (corticosteroids and cyclosporine A) [8]. Researchers are also looking at the link between the common pathogenesis of AD and cardiac diseases initiating inflammatory factors and interleukins and palimony markers. It has long been known that the inflammatory process visible on the skin is the tip of the iceberg. Even in seemingly healthy skin, you can find signs of inflammation and changes in the composition of the corneal envelope [12, 13] as well as elevated inflammatory markers in the bloodstream, e.g. TSLP, TARC, and Th2-driven cytokines [14, 15]. It is commonly known that chronic inflammation is the beginning of the cascade of atherosclerotic plaque formation [16]. There are already studies that early intervention in the case of atopic dermatitis may allow for faster recovery from the disease state, protect against the development of other allergic diseases, and may prevent further consequences in adulthood [17]. Therefore, non-invasive methods of assessing the integrity of these connections are sought. We should know that the vascularity of the skin can be changed under the influence of specific dermatological diseases and systemic pathologies. Impaired flow can be a symptom of a variety of diseases and disorders, including diabetes, cancer, and cardiovascular disease, as well as neurodegenerative and autoimmune diseases. During the ongoing inflammatory process, the vascular endothelium is damaged. Dysfunction of microcirculation is one of the earliest stages in the pathogenesis of these diseases. Endothelial dysfunction comprises attenuating endothelium-dependent vasodilation, augmented vasoconstriction, and microvessel structural remodelling throughout the body. Due to the accessibility of its assessment, peripheral microcirculation has been considered an indicator of systemic microvascular function. Skin circulation has gained prominence as an accessible and potentially representative vascular bed for examining the mechanisms of microcirculatory function and dysfunction. Several invasive and non-invasive methods are used in the assessment of microvascular function, but the different methods of vascular function assessment are not interchangeable (Table 1) [18, 19].
One of the methods used is flow mediated skin fluorescence (FMSF). The FMSF technique is based on monitoring the intensity of the reduced form of nicotinamide adenine dinucleotide (NADH) fluorescence from the skin tissue on the forearm as a function of time in response to blocking and releasing blood flow. NADH and its oxidized form (NAD+) play a crucial role in biological systems. The NADH/NAD+ pair is the carrier of electrons in the respiratory chain of each living cell. NADH, unlike NAD+, emits significant fluorescence. The fluorescence from NADH is the strongest component of the overall fluorescence emitted from human skin. The penetration depth of excitation light for NADH (340 nm) in skin tissue is low (about 0.5 mm), therefore a substantial fraction is absorbed by the epidermis and papillary dermis. In these skin regions, the density of blood microvessels is low and the changes in NADH fluorescence depend on the supply of oxygen diffused from deeper layers [20]. The level of NADH depends on the oxygen supply provided by blood circulation. Since, due to physiological factors (mainly skin pigmentation), direct comparison of absolute levels of fluorescence from the skin is a rather useless assessment of circulatory systems, the FMSF method is used in combination with the most common reactivity test called post-occlusive reactive hyperaemia (PORH) [21, 22]. Microcirculation assessment devices that assess its anatomy and functions seem to be excellent methods, allowing for the analysis of its early disorders, which is the first visible stage in the pathogen of cardiovascular diseases.
Aim
The study aimed to examine changes in NADH fluorescence from the epidermis of a forearm measured with the FMSF technique in patients with AD and to investigate whether they are associated with clinical manifestation of the disease.
Material and methods
Patient characteristics
The study involved 13 patients with AD (the study group) and 11 people selected without any atopic and cardiovascular disease (the control group), aged over 18 years. Changes in NADH fluorescence were measured using FMSF on the forearm in response to blocking and releasing blood flow. The results were represented as ischemic (IRmax and IRindex, HS) and hyperaemic response maximum and area under the curve (HRmax and HRindex). The age of the AD group ranged from 19 to 55 years (7 women and 6 men, with a mean age of 27.62 ±11.10 years and 11 healthy volunteers (4 women and 7 men). The age of the control group ranged from 22 to 43 years, with a mean age of 35.36 ±6.38 years. The diagnosis of AD was based on the classification criteria of Hanifin and Rajka published in 1980. Widely recognized as the earliest diagnostic criteria, the 1980 Hanifin and Rajka criteria were primarily developed to clearly define the AD population for research studies [23, 24]. The SCORAD scale (scoring atopic dermatitis) was used to assess the severity of the disease, the mean SCORAD was 39.4 ±16.9 (Table 2). The study conforms to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the local Ethics Committee and informed consent was obtained from all participants prior to the study.
Table 2
Characteristic of the study population. Sex: Men – M; Women – W. Age of onset: Infantile 2–12 months; Post-infantile 1–3, Preschool 3–7 years; School 8–12; Teenage 13–18; Adult ≥ 19 years. SCORAD (0–108): mild < 25; moderate 25–50; severe > 50. EASI (0–72): mild < 7; moderate 7–20; severe > 20. Itching and Sleep Disorders (0–-10): mild < 3; moderate 4–6; severe > 6
Flow mediated skin fluorescence measurements
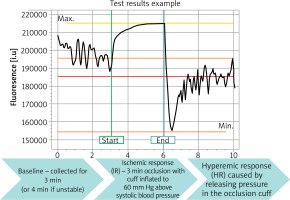
FMSF is a non-invasive optical technique to study microcirculation based on measurements of skin NADH fluorescence intensity. Measurements of NADH were performed with AngioExpert (Angionica, Poland). AngioExpert measures the fluorescence of NADH molecules excited by ultraviolet (UV) radiation of 340 nm (UVB). Since maximum light penetration of 340 nm UVB in the skin is about 0.3 to 0.5 mm, results are determined by fluorescence of NADH within the epidermis. The wavelength of emitted NADH fluorescence (FL) is 460 nm (blue light). The measurement starts at the resting stage, lasting 3 min, with the patient in a comfortable, sitting position, and results are displayed as a baseline level (FL base). This is followed by an occlusion (ischaemic) stage, lasting 3 min, which is caused by the blockage of blood flow in the arm artery with the sphygmomanometer (blood pressure meter). NADH fluorescence is displayed in arbitrary units (a.u.) and reaches a maximum (FL max) in response to ischemia and the accumulation of NADH in keratinocytes. Results are displayed as ischemic response maximum (IRmax) and ischemic response area under the curve (IRindex). Finally, a reperfusion (hyperaemic) stage takes place following sphygmomanometer release: NADH fluorescence rapidly decreases and reaches a minimum (FL min/min) within the first few seconds of reperfusion and then recovers, less steeply, to the baseline level within 5 min. Results are displayed as hyperaemic response max (HRmax) and hyperaemic response area under the curve (HRindex) (Figure 2).
Figure 2
The figure shows a typical FMSF trace. The baseline was collected for 3 min, after which the occlusion cuff was inflated to 60 mm Hg above systolic blood pressure. This resulted in an increase in NADH fluorescence, known as the ischemic (IR) response. After 3 min, the pressure in the cuff was released. The NADH fluorescence fell below baseline, reaching a minimum, followed by a return to baseline. This is known as a hyperaemic response (HR). There are two distinct phases in HR. The first phase, lasting about 20–30 s, is associated with a rapid decrease in NADH fluorescence. The post-congestion stage is followed by reperfusion as NADH fluorescence returns to baseline
Statistical analysis
Statistical analysis was performed with Statistica. The normality of the FMSF parameter distributions was checked using the Shapiro-Wilk test. HRindex, HRmax, and HS are not normally distributed. The significance of intergroup differences in these variables was therefore tested using the Mann-Whitney U test. For the remaining variables, the Student’s t-test was used. Results are represented as median with lower (25th) and upper (75th) quartile (25th–75th centile). In all calculations, a p-value less than 0.05 was regarded as statistically significant.
Results
The mean results of HS, HRindex, IRmax, HRmax, IRindex in patients with atopic dermatitis were 67.66, 10.56, 13.37, 18.25, 10.023 compared to the control 104.12, 12.54, 16.74, 19.29, 12.77 respectively. At the level of statistical significance (p-value of < 0.05), no statistically significant between-group differences were observed. However, a trend was found towards reduced IRmax, IRindex, HRmax, HRindex and HS parameters in patients with atopic dermatitis (Figure 3).
Figure 3
Box-whisker charts showing the minimum value, first quartile, third quartile, and maximum value. The median (bold, black horizontal line) has been marked on the graphs, and the mean (green point) is also marked on the figure of the variable HRindex, HRmax, IRindex and IRmax

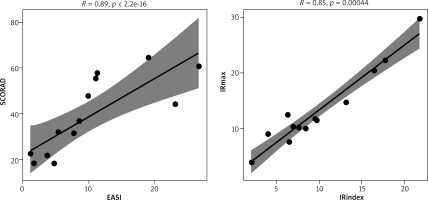
An analysis of correlations between the parameters of circulation and the severity of AD was performed using Spearman’s rank correlation analysis. There was no association between disease severity and NADH fluorescence. Significant and positive correlations occur between selected parameters determining the severity of AD: SCORAD ~ EASI (p << 0.0001), SCORAD ~ sleep disorders (p < 0.05), SCORAD ~ severity of pruritus (p < 0.01), EASI ~ severity of pruritus (p < 0.05). In the case of the parameters of the FMSF technique, a statistically significant and positive correlation occurs between the variables IRmax~IRindex (p << 0.0001) (Figure 4).
Discussion
The FMSF technique is currently being tested for its ability to diagnose various pathologies, disorders, and sports physiology. The FMSF device has been proven to be used to assess microvascular function and metabolic regulation with excellent reproducibility. One of the first clinical trials concerned ischemic heart disease and diabetes. In patients with coronary artery disease (CAD), NADH fluorescence measured by the FMSF device was associated with established plasma endothelial markers. In turn, ischemic and hyperaemic responses were blunted in patients with advanced disease of diabetes [25–28]. Studies of patients with diabetes mellitus type 1 (DM1) have shown that HRindex, HRmax and MR parameters are inversely correlated with age and BMI. The majority of patients with DM1 with HR <8% showed features of dysfunctional metabolic regulation. FMSF appears to be a useful technique for monitoring patients with diabetes over time, allowing for early recognition of potentially dysfunctional microcirculation and metabolic regulation [29]. The FMSF technique demonstrated impaired flow motion responses to hypoxia induced by transient ischemia of diabetic foot ulcer cases, which can be used for identification of types with low prognosis for healing [30]. Similar deviations apply to arterial hypertension (HA), where the early phase of skin ischemia indicates quicker hypoxia-induced NADH accumulation in the skin in patients with HA than in healthy individuals. These findings suggest that some protecting mechanisms postponing the early consequences of early cellular hypoxia and premature NADH accumulation during skin ischemia are impaired in patients with untreated HA [31]. Based on the observed changes in parameters measured with the FMSF technique, it was found that chronic fatigue associated with the post-COVID syndrome is comparable with transient fatigue caused by high-intensity exercise in terms of vascular effects, which are correlated with vascular stress in the macrocirculation and microcirculation [32]. An attempt was made to assess microcirculation function in obstructive pulmonary diseases. Interestingly, the FMSF technique identified microcirculatory disorders in patients with chronic obstructive pulmonary disease (COPD) but not in patients with asthma. The difference may be related to the different age characteristics of patients with asthma, and the coexistence of other diseases in the elderly [33].
For years, there has been a discussion about cutaneous circulation disorders and neurovascular regulation in atopic dermatitis. Interestingly, since the 1980s, dermatologists have been interested in vascular disorders in AD. In the previous centuries, the focus was on clinically visible vascular diseases, such as erythema, oedema, white dermographism or vascular play of atopic skin [34–36]. These patients show marked vasoconstriction after exposure to cold and lower skin adnexal temperature [37]. T cell–endothelial cell interactions are a crucial component to establish acute AD. Various immune cells, including monocytes and mast cells, communicate with the endothelium by releasing inflammatory mediators, thereby stimulating inflammatory mediator release from activated endothelial cells. The process of adhesion, tethering, and transmigration of infiltrating cells is a highly regulated and active communication process between endothelial cells and leukocytes. Thus, the vascular endothelium is in the central regulation of acute and chronic inflammatory responses during AD [38].
This is the first study to use the non-invasive FMSF technique to evaluate NADH fluorescence in patients with AD. The key finding of our pilot study is that the changes of NADH were seen to decrease in both ischemic and reperfusion response in AD patients in comparison to those of healthy control individuals, but it was not statistically significant. Perhaps the differences in NADH levels during the assessment of microcirculation were not sufficiently visible due to the insignificant age differences between the study group and the control group. It cannot be ruled out that despite certain predispositions described in the literature regarding the links between AD and heart disease in young adults, they are not visible enough to demonstrate them. Possibly the pathomechanism of the cooperation of these diseases develops at a different level. Of course, it should be considered that there are no such connections. Further research is needed to confirm or refute these results.
Conclusions
The association of AD with cardiovascular disease is likely multifactorial, involving chronic skin and body inflammation, chronic sleep disorders, decreased physical activity, increased smoking, and alcohol consumption. However, it is not clear which of these risk factors plays the most important role in AD. In addition, it is not known whether the increased cardiovascular risk in patients with AD can be modified. Microvascular endothelial dysfunction may be one of the mechanisms responsible for the increased cardiovascular risk in patients with AD.