Introduction
Fluorescence imaging has become a method for bacterial visualisation in chronic wounds for the last few years [1–4]. It has been demonstrated that a handheld device for fluorescence imaging MolecuLight i:X (MolecuLight, Inc, ON, Canada) visualizes bacterial fluorescence in real time based on spontaneous production of porphyrins and pyoverdines, which fluoresce red and cyan (blue-green), respectively [5–7]. Many clinical trials have shown that the presence of red and/or cyan fluorescence in wounds correlated with moderate-to-heavy bacterial load [1–4]. The device enables quick diagnostics to determine both the type and location of pathogens present in the wound and on the skin. This information is helpful in wound assessment and implementing antimicrobial stewardship interventions even before the microbiological test result is obtained.
The process of healing of a chronic wound is complex and requires adopting multidisciplinary approach. Creating and subsequent maintenance of natural environment and microbiological balance in the wound bed are crucial. An infection in a chronic wound slows down healing, lowers patients’ quality of life (QoL) [8], and increases the costs of healthcare at the same time [9]. The identification of a wound with bacterial colonization poses a considerable challenge since infection may occur even in asymptomatic patients. Apart from biopsy, which is recommended in diagnostic microbiology, bacterial wound culture remains a routine part of standard care. The reliability of the result depends on how precisely the sample was taken from the wound bed and the waiting time is a few days. There are many protocols for detecting “classic symptoms” of bacterial infection, such as pain, absence of healing, purulent exudate, increased exudate, redness, locally elevated temperature and swelling [10–12]. Unfortunately, some chronic wounds do not exhibit typical symptoms of infection and their observation is frequently subjective; moreover, there is a considerable inter-observer variation [13, 14]. Even when the signs and symptoms of infection are present, diagnostic results may not reveal bacterial populations in wound sites [15].
Aim
The aim of this study was microbiological diagnostics of venous ulcer in order to determine the species and location of pathogenic bacteria. Based on the results obtained from fluorescence imaging, targeted topical (antibacterial) actions were implemented. This study also aimed to compare the results obtained from fluorescence imaging (MolecuLight i:X) with the results of bacterial wound cultures obtained in the microbiology laboratory.
Material and methods
The description and stages of the examination performed with the use of MolecuLight i:X device
This study presents two case studies of patients suffering from venous leg ulcers. In both cases it was their first appointment at the Outpatient Department for Chronic Wound Management. After diagnostics had been performed and wound aetiology had been confirmed, clinical signs and symptoms of wound infection were assessed. The assessment was carried out by an interdisciplinary team of experts in wound treatment. Upon removal of the dressing from the wound, the topical treatment involved cleansing the wound bed and surrounding skin with the use of lavaseptic. Then a swab stick was moistened with Natrium Chloratum 0.9% (saline solution) and used to take a sample for microbiological testing [15, 16]. Subsequently, a standard image of the wound was taken, placing the device at 8–12 cm distance from the wound. Standard images of the wound were captured in conventional light setting, then the room was darkened and fluorescence illumination mode was activated (violet light-emitting diodes – LEDs – illuminating field of vision). The device used the range finder to make sure the images were captured within the optimal range (8–12 cm). The light sensor in the device indicated when the room was dark enough to capture fluorescence images. If switching off the light in the room did not result in sufficient darkness, the windows were covered with blinds. All the standard and fluorescent images were taken with the use of the MolecuLight i:X device. The device in question consists of a camera sensor, fluorescence optical emission filter and two light emitting diodes, which emit a narrow band of 405-nm violet-coloured excitation light. Red or cyan fluorescence signals indicate the presence of bacteria at loads > 104 CFU/g (moderate-to-heavy growth) [1, 4, 17]. Red fluorescence is emitted by porphyrins, endogenous fluorophores produced by bacterial species such as Staphylococcus aureus [18]. The cyan fluorescence signal is attributed to pyoverdines, which are uniquely produced by Pseudomonas aeruginosa [19, 20]. These signals are produced by both planktonic as well as biofilm-encased bacteria [18, 21, 22]. The fluorescence signals were displayed on a digital touch screen and immediately interpreted by the clinician. (The procedure with the use of the MolecuLight device was developed based on current literature.) Next, depending on the result obtained from the fluorescence image, appropriate treatment methods were used, such as debridement of the wound surface, disinfection, optimal dressing choice, causal treatment in the form of short-stretch compression bandages. These methods were in accordance with current recommendations for venous ulcer care [23].
Case 1
A 68-year-old female was admitted to the Outpatient Department for Chronic Wound Management with ulceration on the left lower limb.
Medical history: The patient presented with recurrent ulceration that had appeared 3 years earlier, with the first wound appearing 8 years ago. The patient occasionally sought help from GP and attempted to treat the wound on her own, as described below. Despite the recommendations, the patient occasionally used store-bought compression products. Topical cholesterol ointment was used. The wound was cleansed with hydrogen peroxide solution and its edges were secured with zinc oxide topical ointment. The patient did not undergo any diagnostic microbiology tests and did not receive any antibiotics. Pain intensity was rated as 8 on the VAS scale (Visual Analogue Scale). The duplex scan examination revealed superficial insufficiency in superficial (reflux in the saphenous vein), perforating (insufficient Cockett III perforators) and deep veins (post-thrombotic lesions in the popliteal vein).
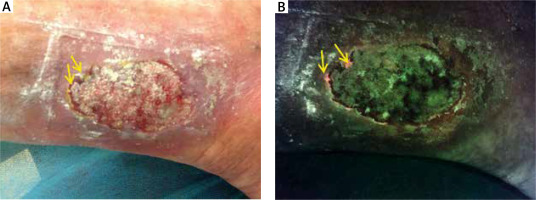
Physical examination: The description of the ulceration: the surface area of the ulceration was 54.75 cm, while its depth was 0.2 cm. The wound bed was covered with granulation tissue in 70% and ointment residue constituted 10% of the surface, particularly in the left lower apex, with the remaining part covered with yellow necrotic tissue. The edges of the wound were clearly demarcated, irregular and slightly undermined in the left apex. The skin surrounding the ulceration was thin and crepey, with features of maceration and saturated hemosiderosis (Figures 1 A, B). There was moderate exudate and the dressing had to be changed once a day, according to the patient. The wound oozed thick yellow odourless discharge.
Figure 1
The images depict case 1 – standard image (A) on the left and fluorescence image (B) on the right, with microbiological culture analysis below. Red fluorescence may be observed, which indicates the presence of bacteria at moderate to heavy loads (yellow arrows). White arrows denote regions of cyan fluorescence. Green colour denotes tissue matrix components [12]. Serratia marcescens 105 CFU/ml (colony-forming unit), Pseudomonas aeruginosa 105 CFU/ml, Gram negative 103 CFU/ml
Ankle-brachial index value on the right lower limb was 1.1 and 1.0 on the left limb.
Treatment: Once the dressing was removed, the surface of the wound and surrounding skin were cleansed mechanically, with the necrotic tissue and blood removed, among others. Dissecting forceps and saline solution were used in order to perform the cleansing. After the wound was cleansed, a sample for microbiological testing was taken. Next, a standard image was taken, followed by a fluorescence image. Finally, the wound was disinfected, thorough wound bed debridement was performed, including its margins and surrounding skin, followed by specialty dressing choice and compression therapy.
Fluorescence imaging revealed areas with increased fluorescence of red and cyan colour in the left upper apex and the centre of the wound. The remaining surface emitted green and dark colour similar to black. Microbiological analysis confirmed the presence of Serratia Marcescens 105 CFU/ml (Colony-Forming Unit), Pseudomonas aeruginosa 105 CFU/ml, Gram-negative bacteria 103 CFU/ml.
Case 2
A 32-year-old male was admitted to the Outpatient Department for Chronic Wound Management with ulceration on the left lower limb.
Medical history: The wound appeared 3 years ago as a result of an injury. So far, the patient received treatment at primary care facility, where ointments and net dressings were used occasionally. No microbiological diagnostic tests were performed and the patient did not receive any oral antibiotics. The patient denied any comorbidities. The patient rated pain intensity as 6 on the VAS scale. The duplex scan examination revealed superficial (reflux in the saphenous vein) and deep (reflux in the popliteal vein) venous pathology.
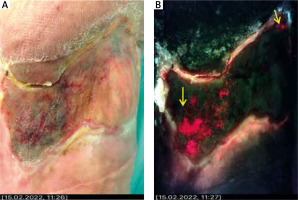
Physical examination: The description of the ulceration: the wound was located on the inner shin of the left lower limb. The surface area of the ulceration was 152 cm, while its depth was 1.5 cm. The wound bed was covered with granulation tissue in 60%, green discoloured tissue (lower and upper inner apex of the wound) constituted 20% of the surface, with the remaining part covered with yellow necrotic tissue. The edges of the wound were irregular, undermined and swollen outward. The skin surrounding the ulceration showed features of maceration, particularly in the lower apex (Figures 2 A, B).
Figure 2
The images depict case 2 – standard image (A) on the left and fluorescence image (B) on the right, with microbiological culture analysis below. Yellow arrows indicate the regions of red fluorescence from bacteria, suggesting the presence of bacteria at moderate to heavy loads. Green colour denotes tissue matrix components [12]. Streptococcus dysgalactiae 103 CFU/ml, Gram-negative 104 CFU/ml, Coagulase-negative Staphylococci 103 CFU/ml
Ankle-brachial index value on the right and left lower limb was 1.2.
The previously established and described procedure was implemented: cleansing of the wound surface, taking sample for microbiological testing, taking standard and fluorescence images. The fluorescence image revealed increased red colour emission, particularly in the lower and right upper apex. Microbiological lab analysis confirmed the presence of Streptococcus dysgalactiae 103 CFU/ml, Gram-negative bacteria 104 CFU/ml, Coagulase-negative staphylococci 103 CFU/ml.
Discussion
Bacteria present in chronic wounds are invisible to the naked eye, yet they may lead to delayed wound healing. Nutrient-rich, moist environment of a chronic wound constitutes perfect breeding ground for pathogenic bacteria. Given the right circumstances, the bacteria populating a chronic wound will start a series of consecutive processes, ultimately resulting in an infection. That is why determining the species and amount of the bacteria in wound bed is essential. The most frequently detected species include Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pyogenes, Escherichia coli, and given their virulence, toxin and enzyme production, they belong to the category of the most dangerous pathogens affecting chronic wounds [24, 25]. Quantitative analysis conducted on tissue biopsies is considered the gold standard for detecting high bacterial load in chronic wounds. Still, the majority of wound care professionals are not able to take biopsies from the wound and microbiological analysis is a more costly and time-consuming procedure in comparison to semi-quantitative assessment/analysis of swab samples [21]. Recently, fluorescent light has gained attention, both as a diagnostic and therapeutic tool [18, 26]. MolecuLight i:X (MolecuLight, Canada) is effective for quick detection of bacteria present in the wound and on the patient’s skin in real time [27]. By means of the fluorescent light illumination the tissues populated by pathogenic bacteria produce fluorescent signals of various colours, depending on the species of bacteria present (Table 1). The image also includes the spatial pattern of bacterial load, which creates bacterial mapping of the wound and may be used by a clinician for targeted sampling, debridement, removal of bacteria and other methods of wound therapy [28]. Thanks to rapid microbiological diagnostics, proper topical therapy, such as wound bed debridement and application of antimicrobial formulations and dressings, may be implemented before results from microbiology laboratory are obtained [18, 29, 30].
Table 1
Potential source of fluorescence on MolecuLight i:X images [5]
| Colour | Pathogen | Tissue | Confounding factors |
|---|---|---|---|
| Red | Porphyrin-producing bacteria1 | – | Ambient light contamination Coloured cleansing solution (for instance, pink chlorhexidine) Red pigment tattoo |
| Cyan | Pseudomonas aeruginosa | – | White cotton (including bed linens, surgical scrubs, clothing) Paper products (for instance, measuring ruler) Blue absorbent pad Blue pigment tattoo |
| White | Pseudomonas aeruginosa | Tendon, bone, flaky skin, nails | White cotton (including bed linens, surgical scrubs, clothing) Paper products (for instance, measuring ruler) Dressings (for instance, gauze, foam bandages) |
| Dark – black | – | Haemoglobin (blood or heavily vascularized tissue) Necrotic tissue | Iodine Silver dressing Gentian violet |
| Green | – | Skin, slough | Collagenase ointment |
1 Including Gram-negative and Gram-positive species, aerobes and anaerobes (for instance, Staphylococcus, E. coli, Klebsiella, Proteus, Enterobacter, Acinetobacter, Aeromonas, Bacteroides, others) [24].
The first example presented a female patient with a venous ulcer. The wound did not exhibit any clear signs of infection. The majority of the wound bed was covered with granulation tissue with some ointment residue and yellow necrotic tissue on the remaining surface. The patient had both standard and fluorescence images taken. Fluorescence image analysis revealed topically increased cyan and red signal emissions, with the majority of the surface emitting green. Green colour is commonly seen in fluorescent images since tissue autofluorescence is mainly caused by endogenous fluorophores, which are components present in tissues that come from extracellular matrix proteins (for instance, collagen, elastin, fibrin). The tissue components generally emits fluoresce green and yellow signals spanning the visible spectrum [5]. In many cases, including ours, cyan fluorescence, which is indicative of Pseudomonas aeruginosa presence, was detected in wounds without typical signs of infection by this pathogen [17, 19, 31]. Venous leg ulcers are the most common chronic wounds, in which cyan fluorescence was detected. It corresponds to known high incidence of Pseudomonas aeruginosa on the surface of venous ulcers [32, 33]. As demonstrated in one study [19], a 78-year-old female patient presented with a venous leg ulcer, with no overt signs and symptoms of infection; as a result, antimicrobial therapy was discontinued. Unfortunately, the wound gradually deteriorated. The patient was referred to the wound care clinic, where initial examination did not detect any infection in the wound. Nevertheless, fluorescence images using the MolecuLight device prompted the clinician to perform antimicrobial interventions, such as precise and thorough wound debridement. In another recently published FLAAG clinical trial/study, the MolecuLight procedure confirmed significantly increased detection of bacteria in non-healing wounds by fourfold. Moreover, fluorescence images detected increased bacterial load in 46% of wounds, which were clinically negative [17]. The study by Serena et al. demonstrated that only 4 out of 19 wounds were positive for clinical signs and symptoms indicative of bacterial load. Combining the observation of signs and symptoms of infection (NERDS: non-healing, exudate, red and bleeding wound surface granulation tissue, debris (discoloration, yellow or black necrotic tissue) and smell or unpleasant odour; STONEES: size increasing, temperature elevation, os (probes to bone), new or satellite areas of breakdown, erythema/oedema, exudate and smell) [34, 35] with fluorescence imaging resulted in improved accuracy from 26.3% to 73.7% and enhanced sensitivity from 22.2% to 72.2%. The authors reported that the use of fluorescence imaging in real time enabled the detection of bacteria without delay and led to immediate interventions such as mechanical debridement in order to reduce bacterial burden [1].
In the second example described, the wound showed signs and symptoms of infection, such as greenish tissue colour or unpleasant smell. The image taken with the use of fluorescence light illumination confirmed the initial diagnosis. The results from microbiology laboratory confirmed the presence of pathogenic bacteria (Streptococcus dysgalactiae 103 CFU/ml, Gram negative 104 CFU/ml, Coagulase-negative Staphylococci 103 CFU/ml). Thanks to early diagnosis, the implementation of appropriate antimicrobial therapies associated with wound hygiene or TIMERS strategy (T – tissue debridement, I – infection and inflammation control, M – moisture balance, E – epidermization stimulation, R – repair and regeneration, S – social and individual-related factors) was possible. Our observations are in accordance with reports from numerous facilities [1, 3, 17], confirming the benefits of fluorescence imaging in terms of early microbiological diagnostics of chronic wounds, including venous ulcers.
The weakness of this study is, according to the authors, a limited number of analyses performed. We have met the standards to ensure optimal research conditions [5], still, we are aware of the fact that the authors are less experienced in comparison to other research facilities. The authors report [5] that clinical experience and reliability in conducting research guarantee accurate and effective interpretation of the fluorescence imaging results.
Conclusions
Contemporary advanced diagnostic techniques, including the use of fluorescent light illumination, complement the microbiological diagnostics of the wound and broaden its assessment when infection is suspected. Early identification of bacterial colonization remains crucial in the treatment of chronic wounds. In particular, the detection of alert pathogens facilitates the implementation of proper antimicrobial stewardship interventions, which inhibit further progress of infection, healing disturbances and other complications.








