INTRODUCTION
Botulinum toxin type A (BoNT-A) is an effective medication for the treatment of a wide range of dermatological and neurological conditions, including facial wrinkles (FW) and chronic migraine (CM) [1]. This toxic protein is originally produced by the bacterium Clostridium botulinum and is able to temporarily paralyze muscles by blocking the release of acetylcholine, a neurotransmitter involved in muscle contrac-tion [2]. This muscle relaxation effect is used to treat wrinkles and is characterized by high efficacy, high patient satisfaction, low side effects, and the absence of a surgical approach [2]. The U.S. Food and Drug Administration (FDA) approved the use of botulinum toxin for the treatment of glabellar lines (GL) in 2002, lateral canthal lines (LCL; commonly referred to as crow’s feet) in 2013, and forehead lines (FL) in 2017 [3].
The first effects of BoNT-A in the treatment of headaches were noticed in the 1990s not by neurologists but by aesthetic physicians, who observed pain relief after treating frown lines with this drug [4]. In 2010, onabotulinumtoxinA (BoNT-A, BOTOX®, Allergan, Irvine, CA, USA) was approved by the FDA for the treatment of chronic migraine (CM) and is the only botulinum biologic approved to date for the preventive treatment of this disabling condition.
The only approved injection protocol for the treatment of migraine with BoNT-A is the Phase III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) paradigm, which involves the administration of BoNT-A at a dose of 155-195 units (U) in 31-39 injection sites, with 5 U in each site, to seven specific muscle groups of the face, head and neck in the glabella and forehead areas [5–7]. This procedure within the upper face is a borderline between neurology and aesthetic medicine. In many countries, CM procedures are performed by professionals with little or no knowledge of neurology but extensive experience in treating the face to improve the patient’s appearance [8].
Not all injectors receive uniform and standardized training that covers issues related to the correct technique of the procedure according to the PREEMPT paradigm, the functional anatomy of the face, and the effects of the neurological and aesthetic use of BoNT-A.
In Poland, CM treatment with BoNT-A is reimbursed under the National Health Fund drug program, which is well known among Polish neurologists [9, 10]. Despite the available information on CM treatment, some aesthetic physicians and patients themselves do not have sufficient knowledge of the correct procedure and are not aware of the risk of failure of this therapy if it is not performed according to the PREEMPT protocol at appropriate intervals and dosage [8, 11].
People around the world choose to treat their wrinkles with BoNT-A. But is treating wrinkles the only reason for having this procedure? We suspect that headaches may be also the motivation for seeing an aesthetic physician for BoNT-A wrinkle injections. Given this context, the question arises as to whether this off-protocol use is effective for the management of headaches, and whether its application is clinically justified. Other questions also arise: Why do patients with headaches not go to a neurologist for treatment according to established standards? Do they prefer to kill two birds with one stone and eliminating two problems at once? How many of these patients have migraine? Would such a headache patient be qualified for BoNT-A treatment by a neurologist? It is possible that these individuals seek consultation with an aesthetic medicine specialist due to not fulfilling the clinical criteria for botulinum toxin type A (BoNT-A) therapy for headache management.
OBJECTIVE
We decided to conduct this pilot study to evaluate the real situation and try to answer the above questions.
The aim of the study was to assess the reasons for seeking treatment for wrinkles with BoNT-A in an aesthetic medicine clinic, taking into account the potential desire for headache relief. The second aim of this study was to answer the question of whether patients undergoing wrinkle treatment in aesthetic medicine clinics experience headache improvement and whether this is a reason to continue this therapy. The results of our study may be a valuable indication for conducting larger trials and evaluating this situation also in other countries to help patients who are not properly implementing CM treatment with BoNT-A.
MATERIAL AND METHODS
This pilot study was conducted between April 2024 and June 2024 among consecutive patients admitted to the Clinic of Aesthetic and Anti-Aging Medicine of the Dr. Boczarska-Jedynak Health Institute in Oświęcim for the treatment of facial wrinkles with BoNT-A.
Clinical data were collected during the patient’s medical interview as a part of the routine management of the patient, and the treatment procedures were in accordance with the BoNT-A summary of product characteristics. Hence, ethical approval was not necessary for the preparation of this article. All patients agreed to provide information about headaches during medical interviews for study purposes. A detailed structured medical interview with a special interest in headaches was conducted by the treating physician (MBJ, HK, AK, MP, AG) as a structured list of questions and answers to be selected by the patients. Patients had the opportunity to omit questions that they felt did not apply to them (e.g., because they did not have headaches) or that they did not want to or could not answer. Therefore, each question had a different number of responses, which was considered in the analysis of the results. Results are presented as percentages of patients.
The injections of BoNT-A were carried out by doctors certified in aesthetic medicine (MBJ, HK, AK, MP, AG). MBJ is also a neurologist with a special interest in headache medicine. The indication for treatment was anti-wrinkle therapy only, and the presence and severity of the headaches did not affect the patient’s eligibility for BoNT-A.
Each patient was treated with BoNT-A (BOTOX®, Allergan, Irvine, CA, USA) in a dilution of 2.5 ml of 0.9% NaCl/100 units (U) at the following doses according to individual anatomical conditions, 10–16 U for FL, 10–16 U for GL, 20–28 U for LCL. The maximum dose was 60 units.
RESULTS
Participants
Ninety-seven consecutive patients (all women) aged 24 to 78 years were included in the study. BoNT-A was administered to reduce GL in 89.6% (n = 86) of patients, FL in 71.9% (n = 69) of patients, and LCL in 62.5% (n = 60) of patients.
All patients had been previously treated with BoNT-A. 51% (n = 49) had been treated every 6–12 months, 25% (n = 24) every 3–6 months, and 3.1% (n = 3) regularly every 3 months, while 20.8% (n = 20) had been treated less than once a year.
Patients had a variable number of previous treatment sessions. 48.5% (n = 47) had 1–3 sessions, 26.8% (n = 26) 4–5 sessions, 18.6% (n = 18) more than 5 sessions, and 6.2% (n = 6) more than 10 sessions.
The clinical characteristics of headaches and results of previous treatment in the study group are shown in table 1. 66% (n = 64) of the patients had suffered from headaches before starting wrinkle treatment with BoNT-A, but only 19.6% (n = 19) of the patients were diagnosed with migraine, 8.2% (n = 8) with tension-type headaches, and 2.1% (n = 2) with cervical headaches (table 1).
Table 1
Clinical characteristics of headaches. Data are expressed as the number (n) of patients responding to each question.
| Characteristics | n (%) |
|---|---|
| Previous headache | |
| Yes | 64 (66.0) |
| No | 33 (34.0) |
| Headache location | |
| Frontal | 46 (47.4) |
| Temporal | 27 (27.8) |
| Orbital | 19 (19.6) |
| Occipital | 6 (6.2) |
| Neck | 6 (6.2) |
| Right-sided | 8 (8.2) |
| Left-sided | 7 (7.2) |
| All head | 4 (4.1) |
| Nature of pain | |
| Dull/pressure | 30 (30.9) |
| Pulsating | 27 (27.8) |
| Compressive | 24 (24.7) |
| Paroxysmal | 15 (15.5) |
| Constant | 14 (14.4) |
| Stabbing | 6 (6.2) |
| Tingling | 6 (6.2) |
| Bursting | 4 (4.1) |
| Electric shock-like | 2 (2.1) |
| Type of aura | |
| Vertigo or dizziness | 16 (19.5) |
| Scintillating scotoma | 13 (15.8) |
| Dark curtain | 7 (8.5) |
| Difficulty or loss of speech | 6 (7.3) |
| Facial or tongue numbness | 5 (6.1) |
| Limb numbness | 4 (4.9) |
| Time to headache after aura | |
| Up to 15 minutes | 5 (5.4) |
| 15–30 minutes | 7 (7.6) |
| 30–60 minutes | 6 (6.5) |
| Over 60 minutes | 6 (6.5) |
| Duration of aura | |
| Up to 15 minutes | 4 (4.4) |
| 15–30 minutes | 9 (9.9) |
| 30–60 minutes | 4 (4.4) |
| Over 60 minutes | 6 (6.6) |
| Acute treatment of headache | |
| OTC medications | 43 (44.3) |
| Rx medications | 15 (15.5) |
| None | 17 (17.5) |
| Accompanying symptoms | |
| Photophobia | 37 (38.5) |
| Phonophobia | 31 (32.3) |
| Rising with activity | 28 (29.2) |
| Nausea | 24 (25) |
| Vomiting | 11 (11.5) |
| Hypersensitivity to touch | 7 (7.3) |
| Tearing | 7 (7.3) |
| Runny nose | 2 (2.1) |
| Facial redness | 2 (2.1) |
| Edema | 1 (1) |
| Time of headache onset | |
| During day | 46 (47.4) |
| Morning | 20 (20.6) |
| Evening | 19 (19.6) |
| At night | 5 (5.2) |
| Duration of the headache* | |
| < 4 hours 30 | (30.9) |
| > 4 hours 35 | (36.1) |
| Headache intensity in NRS scale 1–10 | |
| 1 | 0 |
| 2 | 2 (2.1) |
| 3 | 7 (7.2) |
| 4 | 10 (10.3) |
| 5 | 6 (6.2) |
| 6 | 7 (7.2) |
| 7 | 12 (12.4) |
| 8 | 12 (12.4) |
| 9 | 6 (6.2) |
| 10 | 6 (6.2) |
| Frequency of headaches | |
| > 15 days/month | 6 (6.3) |
| 8–14 days/month | 3 (3.1) |
| 4–7 days/month | 13 (13.5) |
| 0–3 days/month | 24 (25) |
| Once every few months | 27 (28.1) |
| Once a year | 1 (1) |
| Less than once a year | 1 (1) |
| Family history of headaches | |
| Yes | 43 (44.3) |
| No | 54 (55.7) |
52.1% (n = 50) of patients did not contact their general practitioner (GP) despite the headaches, 8.3% (n = 8) did but did not receive a headache diagnosis or treatment, and 4.2% (n = 4) did not have previous satisfactory headache treatment results (table 2). Only 7.3% (n = 7) regularly sought treatment for headaches in the primary care setting (table 2). 39.8% (n = 37) of patients were not seen by a neurologist because they did not know how to get an appointment, and 8.6% (n = 8) had difficulties contacting the neurologist. 10.8% (n = 10) were treated by a neurologist but without satisfactory results, and only 7.5% (n = 7) of patients received regular satisfactory neurological treatment (table 2).
Table 2
Previous headache diagnosis and treatment
Reason for treatment with botulinum toxin type A
49.5% (n = 48) of patients sought treatment solely for wrinkle removal without any interest in headache relief. 45.4% (n = 44) of patients wanted to eliminate wrinkles, and headache relief was also an important factor. 5.2% (n = 5) sought treatment primarily for headache relief, with wrinkle reduction as a secondary goal.
Headaches after wrinkle treatment with botulinum toxin type A
After receiving BoNT-A, 34.7% (n = 33) of patients had the same level of headaches as before. In a quarter of patients, headaches increased after treatment with BoNT-A. 21.1% (n = 20) of patients experienced headaches up to 24 hours after BoNT-A treatment, and 4.2% (n = 4) of patients experienced headaches more frequently after treatment with BoNT-A.
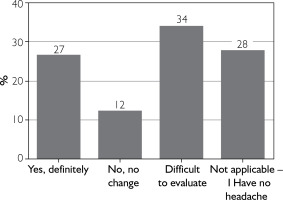
26.8% (n = 26) of patients reported definite relief of headaches after treatment of wrinkles with BoNT-A. 34% (n = 33) had difficulty in assessing the effect, and 12.4% (n = 12) had no change in their headaches (fig. 1).
Figure 1
Answers to the question: “Do you experience headache reduction after administering botulinum toxin for wrinkles?”
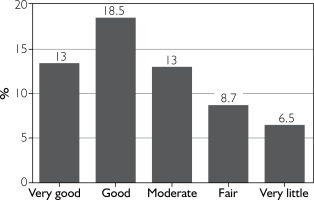
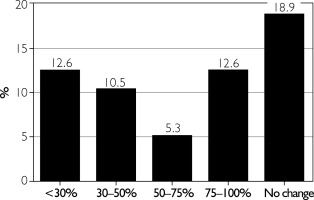
The effect of BoNT-A on headache reduction was rated by patients on a 5-point scale (from very good to little) and is shown in figure 2. Only 18% of patients reported at least a 50% reduction in headache frequency (fig. 3).
Duration of botulinum toxin type A for headaches after wrinkle treatment
22.6% (n = 21) of patients reported the same duration of BoNT-A treatment for wrinkles and headaches. 24.7% (n = 23) of patients claimed that the effect on wrinkles was longer than for headaches. 8.6% (n = 8) of patients experienced a longer effect of BoNT-A on headaches than wrinkles.
Awareness of botulinum toxin type A treatment for migraine
Patient awareness of treatment with BoNT-A for headaches is shown in table 3.
Table 3
Awareness of BoNT-A treatment for headaches and preference of the doctor’s specialty
40.6% (n = 39) of patients were aware of and interested in BoNT-A treatment for migraine. 32.3% (n = 31) were aware but not interested and did not know any details about this type of therapy. 24% (n = 23) had never heard of this option. 3.1% (n = 3) of patients had previously received BoNT-A for migraine.
Preference for treatment by a neurologist or a specialist in aesthetic medicine
When asked: “Would you prefer to have your headaches treated with botulinum toxin by a neurologist and your wrinkles treated by an aesthetic doctor?”, patients responded as follows: 27.1% (n = 26) agreed that headaches should be treated by a neurologist, and 24% (n = 23) preferred to be treated by the same doctor for both headaches and wrinkles (table 3). For 12.5% (n = 12) of patients, the specialty of the doctor did not matter as long as headaches improved. 8.3% (n = 8) of patients felt the specialty of the doctor was not important as long as the wrinkles disappeared (table 3).
DISCUSSION
Botulinum toxin type A – the link between aesthetic and headache medicine
For 30 years, there has been an inextricable link between the treatment of wrinkles and headaches with BoNT-A. The beneficial effect of BoNT-A on headaches was discovered by chance in the 1990s by plastic surgeon Dr William Binder, who was studying the ability of BoNT-A to eliminate facial wrinkles and made a serendipitous discovery; “Instead of finding out what was happening to their wrinkles, many of my patients came back and said, ‘Not only are my wrinkles gone, but my headaches are gone too” [4].
In 1999, Carruthers et al. reported the concomitant improvement of headaches in 9 out of 134 patients as an unexpected clinical benefit of BoNT-A treatment of FL and GL [12]. All these patients were retrospectively diagnosed as having tension-type headache (TTH). They received 12 to 40 units of BoNT-A (BOTOX, Allergan, Inc; Irvine, CA, USA) injected into the glabella and adjacent forehead area for the cosmetic treatment of facial wrinkles [12]. The maximum duration of the clinical benefit for headaches was 8 weeks [12].
Currently, CM is the only approved indication for BoNT-A in headache medicine. Treatment of other types of headaches with BoNT-A is an off-label therapy without proven efficacy. The only BoNT-A for the treatment of CM is BoNT-A, which was approved by the FDA in 2010. BoNT-A is a class A medication recommended by all scientific societies as a first-line treatment for CM [13–15].
The efficacy and safety of BoNT-A in CM using the unified PREEMPT paradigm have been validated in clinical trials, the 15-month PREEMPT trials, the 2-year COMPEL trial, the head-to-head FORWARD trial, and the real-world REPOSE and Chronic Migraine Post-Authorization Safety Study (CM-PASS) trials [5, 6, 16–20]. Since 2010, real-world data have supported the efficacy and safety observed in pivotal randomized controlled trials (RCTs) and long-term open-label studies [21].
Over the past decade, the understanding of the mechanism of action of BoNT-A in headaches has increased significantly. Initially, the effect on headaches was thought to be due to the relaxation of the temporal and frontal-occipital muscles. It is now known that BoNT-A has a significant and separate effect on the nerves, which is crucial in the treatment of pain [22]. The goal of BoNT-A treatment in CM is not muscle relaxation but to reach the nerve endings of the trigeminal-occipital-cervical nerve complex by inhibiting the secretion of pain neurotransmitters [22]. BoNT-A has a unique neuromodulatory effect, leading to peripheral and central desensitization of nerve pathways involved in the pathogenesis of migraine pain [22, 23].
The area of BoNT-A injections in the upper face in CM includes the endings of the first branch of the trigeminal nerve, while the treatment of FL, GL, and LCL includes the first and second branches of the same nerve.
Chronic migraine underdiagnosed and undertreated
CM is a debilitating disorder affecting 1–2% of the world’s population with a significant socioeconomic impact and is defined as the presence of the headache (tension and/or migraine type) on at least 15 days per month during the previous 3 months, fulfilling the criteria for migraine with or without aura on at least 8 days per month and having migraine-like characteristics at onset and responding to triptans or ergot derivatives [24, 25]. The clinical diagnosis could be established by any health professional, including a general practitioner (GP) or an aesthetician, but preferably a neurologist, according to the criteria of the International Classification of Headache Disorders [25].
Unfortunately, although approximately 88% of people with CM worldwide seek help from a healthcare professional, the majority of patients are underdiagnosed. Only 20% of CM patients receive a diagnosis of either CM, chronic daily headache, or transformed migraine [26].
Headaches were common in our group of aesthetic patients, affecting 66% of individuals. Of these, 19.6% met the diagnostic criteria for migraine, but we did not differentiate between episodic and chronic migraine. More than half of our headache patients had not consulted a GP and almost 40% had not even thought of contacting a neurologist about their condition. Only a minority of headache patients were satisfactorily treated by their GP (7.3%) or neurologist (7.5%).
Botulinum toxin type A is approved for chronic migraine, not for other headaches
As mentioned above, CM is the only FDA-approved indication for the treatment of any headache. Despite many reports of the beneficial effects of BoNT-A in the treatment of episodic migraine, TTH, cervicogenic headache, neuralgia, and other headaches, its efficacy has not yet been confirmed in RCTs, and its use is off-label for any other pain indication.
BoNT-A is now widely available to patients with CM on the commercial market and under the Polish Health System/Polish National Health Fund reimbursed drug program [10, 27]. Many people who do not meet the inclusion criteria (e.g., lack of oral treatment failure) or who prefer to be treated in private clinics receive BoNT-A commercially as part of their out-of-pocket expenditure [8]. Despite the extensive information provided by neurologists and the possibilities of CM treatment with BoNT-A, many patients still choose to have their headaches treated in aesthetic medicine clinics under the guise of wrinkle reduction. In support of this hypothesis, headache relief was significantly important for almost half of our patients treated with BoNT-A for wrinkles (45.4%), and headache reduction was a hidden goal for 5.2% of treated patients. This is a large group of patients expecting treatment goals other than just wrinkle reduction.
The need for proper technique
The therapeutic success of BoNT-A treatment in CM depends on the proper qualification of the patient for the procedure and adherence to a strictly defined protocol, including a specific injection technique, drug dose, and treatment intervals [7]. To achieve a beneficial longterm effect, it is necessary to monitor and review the patient’s clinical condition systematically and to control the acute medications used concomitantly [7].
The technique for administering BoNT-A according to the PREEMPT protocol has been described in detail and is widely available to healthcare professionals [7, 8]. BoNT-A should be administered to 31–39 sites in the head and neck area, with the needle inserted shallowly under the skin and 5 U of BoNTA administered at each site. The minimum dose of BoNT-A is 155 U, corresponding to 31 injection sites (approved in both the USA and Europe), and the maximum dose is 195 U, corresponding to 39 injection sites (European approval) [7]. The drug is applied to areas similar to those targeted in wrinkle treatments. However, significant deviations from the established protocol, including variations in dilution, prompted Boczarska-Jedynak and Blumenfeld to publish findings to improve the PREEMPT protocol’s outcomes. Their recommendations specifically address aesthetic considerations in the treatment of the upper face [28].
According to the author’s recent survey of Polish aesthetic physicians, almost half (45.7%) use BoNT-A injections to treat CM, but only 29.1% use BoNT-A according to the PREEMPT protocol [8].
The low success rate in headache reduction in our patients being treated for wrinkles could be due to several reasons. The maximum dose received for wrinkles was 60 U, and the minimum dose for CM in the PREEMPT protocol, as noted above, is 155 U, so the lack of benefit for those with CM receiving wrinkle reduction treatment may be due to an inadequate dose. However, multiple studies of BoNT-A therapy that did not use the PREEMPT protocol failed, and whether this was due to an incorrect therapeutic protocol of injection or due to too low a dose is unknown. Our patients did not receive the PREEMPT protocol as they received BoNT-A for aesthetic reasons. Finally, patients in our study were not assigned an International Classification of Headache Disorders-3 diagnosis, so some may not have had migraine or chronic migraine, with BoNT-A effects unknown or already proven ineffective for other headache types.
In our study, over 70% of our patients were familiar with BoNT-A treatment for headaches, but only 40% had previously been interested in this topic. 3% had previously been treated with BoNT-A for migraine.
The need for repetitive treatment
The cosmetic effect of BoNT-A for wrinkles generally begins within a few days of injection and reaches the maximum effect within 4 weeks [28]. Thereafter, the effect gradually diminishes, and the frown lines eventually return to their original appearance, with patients requiring retreatment to re-establish the effect. In general, the duration of BoNT-A effect after on-label treatment is 3–5 months. Treatment of CM with BoNTA needs to be repeated at least 3 times every 12 weeks to evaluate the clinical effect and can be continued in the case of a good outcome (i.e., at least 50% reduction of headache intensity or frequency) [7].
According to our patients, the effect on wrinkles is longer lasting than on headaches (24.7%) or the same (22.6%). Only 8.6% felt that the analgesic effect was longer.
The possible placebo effect of improper use of botulinum toxin type A in headaches
Despite the awareness and availability of BoNT-A in CM among neurologists, there are still many patients who are receiving this medication inappropriately in aesthetic medicine clinics. This can happen under the guise of treating wrinkles, when, in fact, the reason for using BoNT-A is to improve headaches. There are several reasons why this may occur, including the patient's lack of awareness of the indications for BoNT-A treatment, lack of proper diagnosis and treatment of the patient’s headaches, misinformation from the internet, the lower price of BoNT-A treatment for wrinkles than for migraines (if treated outside the Polish National Health Fund), the lower number of injections, and the lower dosage of the drug for wrinkles than for migraine.
One of the main criticisms of the PREEMPT trials is the high placebo effect observed in both trials. Placebo rates in migraine prophylaxis ranged from 20% to 49% [29]. Nevertheless, BoNT-A appeared statistically superior to placebo on many efficacy variables. However, statistical superiority does not necessarily correlate with clinical improvement. It is unclear when a statistically significant change is equated with a clear clinical benefit. The primary endpoint of the PREEMPT-1 study, headache episodes, may not be an appropriate outcome measure in patients with almost daily headaches [5]. Data from the PREEMPT trials suggest that BoNT-A reduces headache days and improves functioning, quality of life, and patient satisfaction [29].
Patients treated with BoNT-A for cosmetic indications generally report high satisfaction with treatment [30]. 26.8% of our patients achieved good results for headaches during wrinkle treatment, but almost the same number of patients (25.3%) experienced more frequent headaches after BoNT-A injections. One-third of our patients could not assess the effect properly.
Conversely, aesthetic practitioners who are not trained neurologists typically focus solely on the treatment of wrinkles and do not address headaches unless they have received specialized training. If a patient’s underlying goal is to alleviate headaches rather than improve aesthetic concerns, the treatment is unlikely to be satisfactory, as the protocol used is not appropriate for their condition and has a low probability of success. Therefore, it is essential to accurately determine the treatment objective and, when necessary, refer the patient to a qualified specialist for appropriate management of migraines or other headache disorders.
Who should treat patients seeking treatment for both wrinkles and headaches?
Approximately 50% of patients prefer the same physician to administer BoNT-A treatment for both headaches and wrinkles, while about 25% believe this responsibility should lie specifically with a neurologist. This means that neurologists who use BoNT-A for migraine should be familiar with a different technique for administering the same drug for wrinkles [28]. If they are not, patients with headaches will continue to visit aesthetic medicine clinics and use the drug for other indications, hoping that the beneficial aesthetic effect will be accompanied by an effect on headaches.
Another approach would be training practitioners who administer BoNT-A for other therapeutic areas in both proper ICHD3 diagnosis of migraine and CM and in the administration of the PREEMPT protocol.
Bias
Our study has several limitations. It is only a pilot study from a single center and relies on the willingness of respondents to answer the survey. Despite a high response rate, it is not free from non-response bias. The sample size was relatively small compared to the number of aesthetic patients in Poland, so the exact representation of the respondent population is limited. In addition, the exact diagnosis of headaches was not verified by neurologists. Despite these limitations, the issue we have addressed is important and deserves further research as a similar situation might be observed in other countries.
CONCLUSIONS
Approximately half of the patients seeking aesthetic medicine treatments expect not only wrinkle smoothing but also relief from headaches. Many of these patients harbor a hidden goal that is unattainable due to the lack of efficacy of BoNT-A outside the approved treatment protocol for CM. Although most aesthetic patients are aware of BoNT-A’s use in headache management, they often lack an understanding of the distinctions between aesthetic and neurological treatments.
Notably, only one-third of aesthetic patients treated with BoNT-A for wrinkles experience headache relief, a rate comparable to the placebo effect observed in RCTs of BoNT-A for CM. Aesthetic medicine practitioners must recognize this issue and remain vigilant for indications that a patient’s underlying motivation for wrinkle treatment may be headache management rather than aesthetic enhancement.