Introduction
Hidradenitis suppurativa (HS) is a chronic, debilitating, inflammatory skin disease, which has an enormous influence on patients’ quality of life (QoL) [1]. It is characterized by the formation of deeply seated inflammatory nodules, abscesses, perforating sinuses, and scarring in the intertriginous skin areas, like armpits, skin folds, and anogenital region [2]. Due to constant purulent discharge, unpleasant smell, debilitating pain, and associated itch, HS causes immense QoL impairment and is considered the worst dermatosis [3, 4]. It was proven that the QoL decrease in HS patients is higher or similar to those suffering from congestive heart failure or depression [5]. The assessment of QoL is a crucial part of dermatology and a holistic approach to dermatological patients. Due to very distinctive features of clinical picture of HS and the lack of specific questions, generic dermatology QoL questionnaires, including the Dermatology Life Quality Index (DLQI), cannot adequately reflect patients’ suffering [6]. Currently, there are 4 properly validated HS-specific health-related quality of life (HRQoL) instruments, among them the Hidradenitis Suppurativa Quality of Life-24 (HSQoL-24) questionnaire [1].
Aim
The aim of this study was to perform a translation of the HSQoL-24 instrument to Polish language, and its subsequent validation process.
Material and methods
The process of translation and validation of the Polish version of HSQoL-24 was performed according to international guidelines on the methods to translate health-related quality of life questionnaires [7]. The study was performed in line with guidelines for human studies and the World Medical Association Declaration of Helsinki, and it was accepted by the Ethics Committee of Wroclaw Medical University. Participation in the study was voluntary, and all patients agreed to participate. Demographic data of the patients are shown in Table 1.
Table 1
Patients’ characteristic
Translation
The English version of the HSQoL-24 instrument was obtained from the authors with permission to translate and validate it [8]. The first English-Polish translation was performed by 2 independents translators (KT, MS). Then, those 2 versions were analysed in terms of consistency and wording by a third consultant, a bilingual expert in the field (JCS). Subsequently, a backwards translation was performed by 2 independent experts, who were not familiar with the English version of the questionnaire (KB, LM). The 2 versions were later sent to the authors of the original HSQoL-24 (SEM, LTA), who, after discussion, agreed on the final version of the questionnaire.
Validation
The validation process was conducted on a group of 30 HS patients, who were treated in the department of dermatology. All of them were previously diagnosed with HS by an expert in the field. All the patients were asked to complete the HSQoL-24 questionnaire twice, with an interval of 4 to 5 days. Such a period is thought to be short enough to prevent changes in the actual HS severity and its influence on the patients’ QoL, and long enough to avoid repetition patients’ answers. Moreover, each patient was also asked to complete Polish versions of the Dermatology Life Quality Index (DLQI) [9] and Hidradenitis Suppurativa Quality of Life (HiSQoL) [10] questionnaires for HSQoL-24 convergent validation.
Statistical analysis
The statistical analysis of the data was performed with the use of IBM SPSS Statistics v. 26 (SPSS INC., Chicago, USA) software. The internal consistency of the questionnaire was evaluated with Cronbach α coefficient for the whole questionnaire and for every domain. A Cronbach α coefficient of at least 0.7 was considered as confirmation of the internal consistency, while a value above 0.9 meant very good internal consistency [11]. The correlation between the responses from a single completion to each individual question, as well as to the total score, was established with the Spearman correlation test. The questionnaire reproducibility (test-retest reliability) was assessed by comparison of the 2 responses of each patient with the use of the intraclass correlation coefficient (ICC). Similarly to the internal consistency coefficient, to indicate adequate reproducibility of the results the ICC had to be at least 0.7 [12]. The correlations between results from HSQoL-24 first completion and other QoL questionnaires were calculated. Moreover, answers to each question from the first and the second completion were compared by the Wilcoxon signed-rank test to search for significant differences. A 2-sided p-value ≤ 0.05 was considered to be statistically significant.
Results
The results of the internal consistency calculation of the Polish language version of the HSQoL-24 questionnaire proved that all the items correlated with each other. The Cronbach α coefficient for the Polish version of HSQoL-24 global score of 0.908 reflects excellent internal consistency. Moreover, similar results were obtained for almost all of the questionnaire domains: 0.916 for psychosocial, 0.819 for economic, 0.919 for employment, 0.842 for social interaction, and 0.78 for clinical. Only the personal domain did not present satisfactory internal validity, with a Cronbach α coefficient of 0.460; however, it did not influence the internal consistency of the whole instrument. Positive, significant correlations were found between answers to each question and the HSQoL-24 global score (Table 2). The Spearman correlation coefficient for every item and the total score varied from 0.369 to 0.815, indicating moderate to strong correlations. Only Questions 4 and 22 did not show any correlation with total score, but they correlated positively with other answers (Table 2). All the above-mentioned results demonstrated very good convergent validity of the translated version of the questionnaire.
Table 2
Reproducibility of the results
| Item (mean ± SD) | 1st assessment [points] | 2nd assessment [points] | P-value |
|---|---|---|---|
| Q1 | 1.8 ±1.06 | 1.47 ±1.01 | 0.033* |
| Q2 | 1.5 ±1.01 | 1.43 ±0.97 | 0.653 |
| Q3 | 1.0 ±0.74 | 0.80 ±0.85 | 0.109 |
| Q4 | 1.97 ±0.96 | 1.57 ±1.07 | 0.027 |
| Q5 | 0.87 ±1.20 | 0.6 ±0.81 | 0.033 |
| Q6 | 1.9 ±0.96 | 1.93 ±1.08 | 0.851 |
| Q7 | 0.17 ±0.46 | 0.17 ±0.46 | 1 |
| Q8 | 1.73 ±1.39 | 1.67 ±1.35 | 0.593 |
| Q9 | 2.0 ±1.11 | 1.63 ±1.16 | 0.016* |
| Q10 | 1.47 ±1.14 | 1.57 ±0.97 | 0.499 |
| Q11 | 1.43 ±1.28 | 1.27 ±1.28 | 0.449 |
| Q12 | 1.9 ±1.27 | 1.73 ±1.20 | 0.244 |
| Q13 | 1.97 ±1.33 | 1.63 ±1.30 | 0.083 |
| Q14 | 1.3 ±0.99 | 1.27 ±0.91 | 0.705 |
| Q15 | 0.97 ±1.0 | 0.70 ±0.95 | 0.065 |
| Q16 | 1.43 ±1.07 | 1.2 ±1.06 | 0.108 |
| Q17 | 0.5 ±0.86 | 0.93 ±1.14 | 0.013* |
| Q18 | 1.93 ±1.14 | 1.73 ±1.08 | 0.130 |
| Q19 | 1.67 ±1.35 | 1.70 ±1.32 | 0.830 |
| Q20 | 0.7 ±0.88 | 0.47 ±0.57 | 0.058 |
| Q21 | 1.07 ±0.74 | 1.03 ±0.96 | 0.830 |
| Q22 | 0.93 ±0.98 | 1.13 ±0.90 | 0.243 |
| Q23 | 1.3 ±0.84 | 1.53 ±0.97 | 0.090 |
| Q24 | 2.0 ±1.13 | 1.80 ±1.10 | 0.242 |
| Total score | 33.5 ±14.38 | 30.97 ±13.6 | 0.371 |
The repeatability of the results was assessed with the use of the ICC as 0.908 for the HSQoL-24 global score. Moreover, no differences in most questions were found between first and second completion (Table 3). Only answers to questions 1, 9, and 17 differed between each completion, but it did not interfere with the reproducibility of the questionnaire global score. Moreover, statistically significant, positive correlations were obtained between the answers in the first and second survey (detailed data not shown).
Table 3
Correlations between answers to every question and the total score of Hidradenitis Suppurativa Quality of Life 24 (HSQoL-24)
| Spearman coefficient | Q 1 | Q 2 | Q 3 | Q 4 | Q 5 | Q 6 | Q 7 | Q 8 | Q 9 | Q 10 | Q 11 | Q 12 | Q 13 | Q 14 | Q 15 | Q 16 | Q 17 | Q 18 | Q 19 | Q 20 | Q 21 | Q 22 | Q 23 | Q 24 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q 1 | 1 | ||||||||||||||||||||||||
| Q 2 | 0.179c | 1 | |||||||||||||||||||||||
| Q 3 | 0.735b | 0.360c | 1 | ||||||||||||||||||||||
| Q 4 | 0.521b | 0.252c | 0.491b | 1 | |||||||||||||||||||||
| Q 5 | 0.573b | 0.337c | 0.624b | 0.060c | 1 | ||||||||||||||||||||
| Q 6 | 0.373a | –0.078c | 0.358c | 0.279c | 0.040c | 1 | |||||||||||||||||||
| Q 7 | 0.368a | 0.208c | 0.536b | 0.151c | 0.488b | 0.157c | 1 | ||||||||||||||||||
| Q 8 | 0.395a | 0.441a | 0.408a | 0.235c | 0.449a | 0.181c | 0.265c | 1 | |||||||||||||||||
| Q 9 | 0.417a | 0.312c | 0.375a | 0.193c | 0.254c | 0.284c | 0.249c | 0.604b | 1 | ||||||||||||||||
| Q 10 | 0.377a | –0.078c | 0.294c | –0.087c | 0.514b | 0.178c | 0.394a | 0.212c | 0.111c | 1 | |||||||||||||||
| Q 11 | 0.585b | 0.241c | 0.526b | –0.024c | 0.735b | –0.147c | 0.384a | 0.403a | 0.326c | 0.627b | 1 | ||||||||||||||
| Q 12 | 0.597b | 0.175c | 0.627b | 0.119c | 0.653b | 0.109c | 0.362a | 0.395a | 0.413a | 0.615b | 0.806b | 1 | |||||||||||||
| Q 13 | 0.175c | 0.252c | 0.430a | 0.075c | 0.206c | 0.109c | 0.472b | 0.205c | 0.402a | 0.062c | 0.300c | 0.437a | 1 | ||||||||||||
| Q 14 | 0.483b | 0.183c | 0.458a | 0.124c | 0.493b | 0.453a | 0.468b | 0.328c | 0.500b | 0.102c | 0.279c | 0.317c | 0.507b | 1 | |||||||||||
| Q 15 | 0.473b | 0.320c | 0.563b | 0.135c | 0.516b | 0.189c | 0.448a | 0.507b | 0.586b | 0.127c | 0.515b | 0.506b | 0.710b | 0.805b | 1 | ||||||||||
| Q 16 | 0.631b | 0.170c | 0.505b | 0.072c | 0.697b | 0.287c | 0.386a | 0.375a | 0.411a | 0.609b | 0.674b | 0.693b | 0.204c | 0.421a | 0.460a | 1 | |||||||||
| Q 17 | 0.505b | 0.024c | 0.460a | 0.244c | 0.450a | 0.256c | 0.110c | –0.025c | –0.150c | 0.269c | 0.276c | 0.222c | –0.188c | 0.299c | 0.228c | 0.365a | 1 | ||||||||
| Q 18 | 0.653b | 0.244c | 0.665b | 0.151c | 0.513b | 0.371a | 0.381a | 0.440a | 0.689b | 0.364a | 0.642b | 0.741b | 0.517b | 0.607b | 0.717b | 0.702b | 0.186c | 1 | |||||||
| Q 19 | 0.442a | 0.062c | 0.232c | –0.226c | 0.536b | 0.196c | 0.199c | 0.190c | 0.239c | 0.645b | 0.635b | 0.543b | 0.093c | 0.229c | 0.219c | 0.638b | 0.144c | 0.598b | 1 | ||||||
| Q 20 | 0.124c | 0.144c | 0.245c | 0.074c | 0.183c | 0.219c | 0.089c | 0.437a | 0.541b | 0.198c | 0.264c | 0.214c | 0.256c | 0.473b | 0.559b | 0.221c | 0.147c | 0.437a | 0.016c | 1 | |||||
| Q 21 | 0.246c | 0.056c | 0.185c | –0.078c | 0.481b | 0.091c | 0.241c | –0.066c | 0.105c | 0.161c | 0.310c | 0.415a | 0.198c | 0.557b | 0.310c | 0.444a | 0.230c | 0.310c | 0.359c | 0.017c | 1 | ||||
| Q 22 | 0.348c | –0.057c | 0.446a | 0.385a | 0.135c | 0.442a | 0.114c | 0.050c | 0.109c | 0.174c | 0.027c | 0.148c | 0.012c | 0.036c | 0.080c | 0.126c | 0.448a | 0.181c | 0.037c | 0.043c | –0.103c | 1 | |||
| Q 23 | 0.437a | 0.480b | 0.608b | 0.360c | 0.483b | 0.063c | 0.526b | 0.428a | 0.325c | –0.014c | 0.227c | 0.242c | 0.240c | 0.359c | 0.430a | 0.169c | 0.243c | 0.194c | –0.091c | –0.004c | 0.159c | 0.226c | 1 | ||
| Q 24 | 0.397a | 0.332cc | 0.387a | 0.510b | 0.154c | 0.138c | 0.109c | 0.261c | 0.128c | –0.052c | 0.072c | 0.052c | 0.091c | 0.318c | 0.348c | 0.145c | 0.335c | 0.234c | –0.032c | 0.156c | 0.135c | 0.303c | 0.479b | 1 | |
| Total | 0.804b | 0.369a | 0.815b | 0.339c | 0.780b | 0.361a | 0.520b | 0.593b | 0.595bb | 0.505b | 0.736b | 0.778b | 0.484b | 0.653b | 0.751b | 0.784b | 0.412a | 0.849b | 0.537b | 0.417a | 0.387a | 0.337c | 0.468b | 0.402a | 1 |
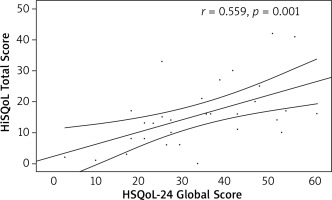
The convergent validity of the Polish version of the questionnaire was confirmed. Statistically significant, positive, moderate correlations were found with DLQI (r = 0.579, p = 0.001) and HiSQoL total score (r = 0.559, p = 0.001) (Figures 1, 2).
Figure 1
Correlation of the Hidradenitis Suppurativa Quality of Life 24 (HSQoL-24) global score and Dermatology Life Quality Index (DLQI) total score
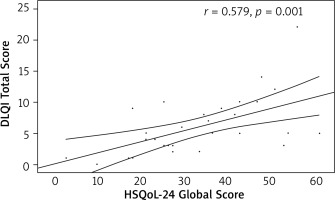
Figure 2
Correlation of the Hidradenitis Suppurativa Quality of Life 24 (HSQoL-24) global score and Hidradenitis Suppurativa Quality of Life (HiSQoL) total score
The Polish version of the validated questionnaire is shown in Supplementary material.
Discussion
The huge Impact of HS on QoL is well-documented [1, 3]. The associated symptoms, frequently embarrassing and troublesome, are directly connected with the feeling of stigmatization, marginalization, and loneliness [13, 14]. HS is associated with high incidence of severe socio-economic problems with decreased work productivity and longer absence [15]. Moreover, it is associated with higher prevalence of sleep impairment or insomnia, sexual problems, depression, alexithymia, and even suicidal thoughts [4, 16–18]. Besides the obvious influence on patients who suffer from HS, the disease also affects their partners and families, and can lead to intimate partner violence and intimidation [19, 20]. Therefore, there is a need for adequate assessment of patients’ QoL impairment and the creation of disease-specific QoL instruments. The HSQoL-24 instrument is a Spanish HS-specific questionnaire developed by Marrón et al. [21] in 2019. It consists of 24 self-administered items evaluating 6 life domains (psychosocial, economic, occupational, relationships, personal, and clinical) in a 4-week recall period. Each item is scored on a 5-point Likert scale (0 = never and 4 = always). Three items [6, 17, 22] are scored inversely. The global score is calculated by adding answers of each score. The maximum score is 96 points, while the minimum is 0 points, and the higher the score, the bigger the effect on the patient’s QoL. The conversion of the score to a percentage requires the multiplication of each score by the domain coefficient: global score: 1.0412; psychosocial: 2.08; economic: 25.0; employment: 12.5; social interaction: 6.25; personal: 12.5; clinical: 8.33. total score: 1.0412; psychosocial: 2.08; economic: 25.0; employment: 12.5; social interaction: 6.25; personal: 12.5; clinical: 8.33 [21]. The questionnaire was translated and validated into English in 2021 by the same group [21].
The Polish version of HSQoL-24 showed comparable but slightly higher internal consistency than the English version with a Cronbach α for global score of 0.908 and 0.866, respectively [21]. Moreover, additional consistency analysis was performed for each domain of the Polish version. The validity of newly translated version was comparable to its English equivalent. Both correlated positively with DLQI with Spearman coefficients of 0.579 for the Polish and 0.690 for the English version (p = 0.001 and p < 0.001, respectively). Although the authors of the original questionnaire performed the second convergent validation with Skindex-29, obtaining strong positive correlation, we used another HS-specific questionnaire. The moderate, positive correlation with HiSQoL total score (r = 0.559, p = 0.001) was obtained, indicating adequate validity of the newly translated instrument.
Our group conducted similar projects for different QoL instruments, obtaining comparable results. In 2020 we translated and validated the HiSQoL instrument, which became the first HS-specific questionnaire in Polish language [10]. HiSQoL showed excellent internal consistency, with a Cronbach α of 0.966 for total score and 0.87–0.94 for 3 subscales [10]. Nevertheless, it is important to underline that HSQoL-24 is more thorough and has a much longer recall period. Previously, Szepietowski et al. [9] performed translation and validation for DLQI, which is currently widely used in research and clinical practice in Poland. The group obtained very good results, with a Cronbach α of 0.9 [9]. Moreover, Polish and Arabic versions of the 6-Item Stigmatization Scale (6-ISS) were created [22, 23]. For both instruments the authors achieved very good results (Cronbach α of 0.94 an 0.89, respectively) [22, 23].
To the best of our knowledge, this is the first translation and validation of the English version of HSQoL-24. Our recently created instrument (Polish language version) showed excellent internal consistency, good reproducibility, and adequate convergent validity. This proves that the Polish version of HSQoL-24 could be of help in assessing QoL impairment in patients suffering from HS in daily clinical practice. Moreover, such a correct process of validation enables researchers to safely use the instrument in dermatological research.








