Introduction
Hidradenitis suppurativa (HS) is an immune-mediated, autoinflammatory skin disease that primarily affects the pilosebaceous follicles and has various clinical manifestations [1]. Determination of the clinical phenotype is important for treatment selection. Multiple phenotypic classifications further refine the understanding of HS, enabling more individualized approaches [2]. Traditional clinical examination may be limited in assessing the full extent of HS lesions, specifically in detecting subclinical lesions. Additional diagnostic modalities improve the evaluation of the disease. High-frequency ultrasound is a non-invasive diagnostic tool that provides detailed visualization of the skin and subcutaneous structures, allowing for the detection of early changes which can influence the disease severity assessments and guide treatment decisions. By offering real-time visualization it can be useful in preoperative mapping, in monitoring disease progression and treatment response [3].
Aim
The aim of the study was to compare the clinical severity stage of HS with the ultrasonography-based staging and to explore the relationships between demographic data, risk factors and clinical phenotypes.
Material and methods
An ongoing pilot study started in 2022, has included 98 inpatients and outpatients of the Bulgarian HS Expert Centre within the Dermatology Clinic of University Multiprofile Hospital for Active Treatment “Prof. Dr. Stoyan Kirkovich”, Stara Zagora, Bulgaria. Participants were selected based on their willingness to undergo clinical and ultrasound evaluations. Informed consent was obtained from all participants before their inclusion in the study. The study was approved by the Ethics Committee of Trakia University and the University Multiprofile Hospital for Active Treatment “Prof. Dr. Stoyan Kirkovich” in accordance with the Helsinki Declaration of 1975, as revised in 2000. A detailed epidemiological study collected data on gender, age, duration of the symptoms, smoking and comorbidities. To assess the duration of HS symptoms, patients were categorised into two groups: short disease duration (SDD, ≤ 5 years) and long disease duration (LDD, > 5 years). Patients were categorised according to the phenotypic classification of Van der Zee and Jemec [4], modified by the Bulgarian HS Expert Centre into regular, follicular, anogenital/sacrogluteal, conglobate, elephantiasis nostra- like, PG-like, cutis verticis gyrata-like, syndromic and mixed phenotype (Table 1). Clinical assessment of the severity of HS was performed using the clinical Hurley staging system, International Hidradenitis Suppurativa Severity Scoring System (IHS4) and Hidradenitis Suppurativa Physician’s Global Assessment scale (HS-PGA). Ultrasound evaluation was done using the ultrasound device MyLab Sigma equipped with a 22 MHz linear probe applying the SOS-HS and ultrasound-based US IHS4 and US HS-PGA staging systems.
Table 1
Van der Zee and Jemec phenotype classification and modified classification
| Van Der Zee and Jemec classification [4] | Modified Van der Zee and Jemec classification |
|---|---|
| Regular | Regular |
| Scarring folliculitis | Follicular |
| Frictional furuncle type | Anogenital/sacrogluteal |
| Conglobata | Conglobate |
| Syndromic | Elephantiasis nostra-like |
| Ectopic | PG-like |
| Cutis verticis gyrate-like | |
| Syndromic | |
| Mixed |
Statistical analysis
The collected data underwent statistical analysis using Kolmogorov-Smirnov and Shapiro-Wilk tests for normality, descriptive statistics, Spearman rank correlation, c2 tests, Wilcoxon signed-rank test, regression analysis, performed by SPSS Statistics 19 and Microsoft Excel 2010. Statistical significance was determined by calculating p-values (p < 0.05).
Results
A total of 98 patients were included in the study. Detailed information on demographics, comorbidities, and clinical phenotypes distribution is summarized in Table 2. The age of the cohort had a normal distribution (p = 0.161 for Kolmogorov-Smirnov and p = 0.137 for Shapiro-Wilk). The mean age was 36.69 years (95% CI: 34.31, 39.07). A predominance of males (73/98, 74.5%) was observed. The mean duration of symptoms was 7.6 years (95% CI: 6.44, 8.88). The disease duration did not follow a normal distribution (Kolmogorov-Smirnov, p < 0.001 and Shapiro-Wilk, p < 0.001). The distribution of patients by duration within the groups was 48/98 (49.0%) patients in SDD and 50/98 (51.0%) patients in the LDD group. The most common HS phenotype in the cohort was the regular (53/98, 53%), followed by the mixed (20/98, 22%) and follicular (13/98, 13%) phenotype (Table 3). Assessment of the HS severity using the Hurley staging system showed that most of the patients were in Stage II (58/98, 59%), while the ultrasound SOS-HS showed a prevalence of Stage III (76/98, 78%) (Table 4).
Table 2
Patient demographic data and comorbidities
Table 3
Phenotype distribution of the patients
| Phenotype distribution | n (%) |
|---|---|
| Regular | 53 (53) |
| Follicular | 13 (13) |
| Anogenital/sacrogluteal | 7 (7) |
| Conglobate | 1 (1) |
| Elephantiasis nostra-like | 1 (1) |
| PG-like | 1 (1) |
| Syndromic | 2 (SAPHO: 1, PASH: 1) (2) |
| Mixed | 20 (22) |
Table 4
Staging of HS: clinical and ultrasonographic assessment across different scales
Potential associations between demographics, comorbidities, clinical, ultrasound assessments, and clinical phenotypes were explored (Table 5).
Table 5
Analysis of associations between disease severity, comorbidities and phenotypes
No statistically significant association was found between gender and disease severity, defined clinically by the Hurley staging system (c2 = 1.506, p = 0.471) and by ultrasound SOS-HS severity (χ2 = 0.046, p = 0.829). The multinomial logistic regression between gender and the clinical HS phenotypes showed no statistically significant association except between the female gender and follicular phenotype of HS (Coef 1.278, p = 0.046, 95% CI: 1.021, 12.620). A positive correlation between age and disease severity according to Hurley stage (r = 0.228, p = 0.024) was established, indicating that higher stages are associated with increasing age. Ordinal regression analysis further confirmed that age significantly impacts disease severity (χ2 = 71.418, R2 = 0.625, p = 0.003). Correlation between age and SOS-HS severity (r = 0.041, p = 0.688) was not found, as well as by ordinal regression analysis (χ2 = 41.577, R2 = 0.528, p = 0.489). No statistically significant association was found between smoking and disease severity, assessed clinically using the Hurley staging system (χ2 = 1.097, p = 0.895) or by ultrasound using the SOS-HS scale. Disease duration was significantly associated with HS severity as assessed by the Hurley stage (χ2 = 7.656, p = 0.022), with a trend noted (χ2 = 6.981, p = 0.008) and confirmed by ordinal regression (PLUM) (χ2 = 6.739, p = 0.009), showing higher severity in those with longer duration (Estimate = –1.078, p = 0.011). No significant association was found when the severity was assessed by ultrasound SOS-HS scale (χ2 = 1.149, p = 0.284), ordinal regression (χ2 = 1.165, p = 0.280). In 54 of the total 98 (55%) patients one or more concomitant diseases were registered (Table 2). The distribution of patients by the duration of complaints and total number of comorbidities was as follows: 48/98 (49.0%) with duration of the complaints in the SDD group had a total of 24 registered comorbidities (M = 0.50, SD = 0.684), while 50/98 (51.0%) in the LDD had 43 comorbidities (M = 0.86, SD = 0.783). The Mann-Whitney U test (U = 878.5, Z = –2.517, p = 0.012) showed significant differences between the groups. The duration of complaints and the number of comorbidities were positively correlated (r = 0.256, p = 0.011).
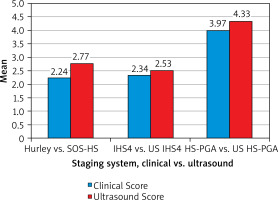
The comparison between clinical and ultrasound severity HS assessments showed statistically significant differences (Figure 1). The ultrasound assessments generally provided higher disease severity compared to clinical evaluation on all scales. The clinical Hurley staging vs. ultrasound SOS-HS showed a significant difference in severity assessments (Wilcoxon Z = –6.814, p < 0.05). Spearman’s correlation demonstrated a positive relationship between the two scales (r = 0.426, p = 0.0001). When we compared the clinical IHS4 vs. ultrasound US IHS4 a significant difference in the two methods was also observed (Wilcoxon Z = –4.025, p < 0.05). Spearman’s correlation revealed a strong positive correlation (r = 0.805, p < 0.05). When comparing the clinical IHS4 severity in points with the US IHS4, the ultrasound points demonstrated significantly higher severity (Wilcoxon Z = –7.066, p < 0.05), with a strong positive correlation between the assessments (r = 0.922, p < 0.05). For the clinical HS-PGA vs. ultrasound US HS-PGA, there was a statistically significant difference (Wilcoxon Z = –4.782, p < 0.05), and the two scales were strongly positively correlated with each other (r = 0.813, p < 0.05).
Comparative analysis between the different phenotypes and clinical and ultrasonographic stages was performed exploring the correlations and differences in scoring systems and assessment methods. For the regular phenotype, significant associations were found between Hurley score and SOS-HS (p < 0.01, r = 0.680), as well as between the IHS4 and US IHS4 (p < 0.01, r = 0.907) and between HS-PGA and US HS-PGA (p < 0.01, r = 0.853). Wilcoxon test showed statistically significant differences between Hurley and SOS-HS (p = 0.008, Z = –2.646), IHS4 and US IHS4 (p = 0.046, Z = –2.000), HS-PGA and US HS-PGA (p = 0.004, Z = –2.889). In patients with follicular phenotype, no significant correlation between Hurley and SOS-HS assessment was determined (r = 0.501, p = 0.081) but a difference was noted (p = 0.002, Z = –3.127). In contrast, correlations between IHS4 and US IHS4 were found (r = 0.853, p < 0.01) as well as between HS-PGA and US HS-PGA (r = 0.707, p < 0.01). For the anogenital phenotype, there were significant correlations between IHS4 and US IHS4 (r = 0.776, p < 0.05), and between HS-PGA and US HS-PGA (r = 0.919, p < 0.01). However, no correlation was found between Hurley and HS-SOS (p = 1.000). No significant correlations or differences were found for the conglobate, elephantiasis nostra-like, PG-like, and syndromic phenotypes due to the small number of patients included in these groups and the rarity of phenotypes. Within the mixed phenotype, notable correlations were seen between Hurley and SOS-HS (r = 0.669, p < 0.01), IHS4 and US IHS4 (r = 0.897, p < 0.01), and HS-PGA and US HS-PGA (r = 0.768, p < 0.01). Significant differences were also noted between Hurley and SOS-HS (p = 0.008, Z = –2.646) and US IHS4 and IHS4 (p = 0.046, Z = –2.000).
Discussion
Our study revealed a male predominance (74.5%) and the mean age of 36.69 years coinciding with reported data that HS typically affects people in their thirties and forties [5]. The mean duration of HS symptoms was 7.6 years, and up to 30 years in some cases. The results are similar to those from our previous study which reported a male predominance (60.4%), a mean age of 32 years, and average symptom duration of 8 years [6]. Our findings align with the reported average diagnostic delay of 10.1 years among 285 patients in a recent study by Aparício Martins et al. [7]. This is probably due to misdiagnosis, the chronic nature of the disease, atypical clinical manifestations and comorbidities. Further, we classified our patients into two groups (SDD: < 5 years and LDD: > 5 years) to investigate the impact of disease duration. This classification was inspired by approaches used in other chronic inflammatory conditions, such as psoriasis, where categorizing patients by disease duration (e.g. SDD: ≤ 2 years and LDD: > 2 years) highlights the benefits of early treatment and its influence on long-term outcomes [8]. In HS, the 5-year cutoff was used in a 2020 study by Marzano et al., which showed that patients with disease duration exceeding 5 years had higher severity scores and HIDRAdisk score compared to those with a shorter disease duration (< 5 years) [9]. More recently, Aparício Martins et al. also applied the same 5-year threshold, demonstrating that delays in diagnosis beyond this period are significantly associated with more severe disease, systemic comorbidities, and poorer outcomes [7]. Real-world data further support the notion that earlier treatment improves outcomes and reduces disease burden [10]. Based on this, we suggest that the 5-year cutoff represents a “window of opportunity” in HS, during which diagnosis and treatment can prevent progression of the disease and optimize the long-term outcomes. This framework may offer a useful way to distinguish between potentially modifiable early-stage disease and more advanced cases. A significant association was found between the female gender and the follicular phenotype and this finding is confirmed by a study of Moata et al. [11]. Follicular lesions observed in the earlier stages of HS may be more common in women as they seek medical care before the disease progresses. Smoking has been established as a major risk factor associated with the pathogenesis of the disease. Results of our cohort found a high percentage of smokers – 61% (60/98) with male predominance (68.3%) and are similar to the cohort study performed by Yüksel and Basım in Turkey which reported that 60.6% (126/208) of their patients were smokers, again with male predominance (74.5%) [12].
In terms of clinical phenotypes, our study found the regular phenotype to be most prevalent followed by the follicular phenotype, completely in line with the literature data [13]. Some rare phenotypes including recently described PG-like, elephantiasis nostra-like and SAPHOSH syndrome (synovitis, acne-pustulosis, hyperostosis, osteitis, suppurative hidradenitis-HS) were recently reported by our team [14, 15], which prompted us to suggest a modification of the Van der Zee and Jemec classification for more accurate categorization of atypical disease presentations [16]. Comparison between clinical Hurley and SOS-HS assessments showed a significant difference in which the ultrasound-based scale demonstrated higher disease severity than the clinical assessment. This is consistent with the findings of Nazzaro et al. that ultrasonography is more sensitive to detecting higher disease severity as compared to the clinical staging alone (k = 0.477). We also found a significant difference between clinical HS-PGA and US HS-PGA scores (3.97 vs. 4.33), once again showing more severe disease staging with ultrasonography which aligns with the data reported by Lacarrubba et al. (2.70 vs. 2.92), and in other studies [17, 18]. Further results from a comparison between clinical IHS4 and equivalent ultrasound IHS4 scoring systems revealed a statistically significant difference with higher disease severity using ultrasonography. To our knowledge, the use of ultrasound-based IHS4 to assess severity has not been previously reported. These findings support our assertion that ultrasound provides a more comprehensive evaluation of disease severity than clinical assessment alone. No statistically significant relationship between gender and disease severity assessed by the Hurley staging system or SOS-HS was found. These data are not in line with other studies that show increased disease severity in males [19], but this may be due to regional differences and age.
A comparison between the age and disease severity using the Hurley stage showed a positive correlation, which is generally in line with other studies indicating that older patients have more severe HS [20, 21], likely due to age-associated alterations in the immune system with chronic inflammation and irreversible structural damage and chronic progressive nature of the disease. Our study established a significant association between disease duration and severity measured by the Hurley staging system. This indicates that longer disease duration is associated with greater severity, consistent with findings from other studies [22]. In our small cohort, we could not find any significant correlation between age and SOS-HS severity, nor between the duration of HS symptoms and ultrasound SOS-HS severity. The duration of the disease was significantly positively correlated with the number of comorbidities, indicating that as severity increases, the number of concomitant diseases also rises, as noted in previous studies [22]. The presence of multiple comorbidities in patients with severe HS necessitates a multidisciplinary approach at HS Expert Centres, ensuring early diagnosis, treatment, and monitoring of the disease. Statistical analysis revealed no significant relationship between severity and the number of comorbidities, whether assessed by clinical Hurley staging or ultrasound SOS-HS. The lack of significance in our findings could be due to the smaller sample size and possible underreporting of comorbidities. Smoking was not significantly associated with disease severity in our cohort either in clinical assessments or by ultrasound. This finding is in agreement with some other studies [23], whereas some suggest a strong relationship between smoking and increased HS severity [24–26]. This discrepancy may be due to differences in factors such as age and regional characteristics that may influence the association between smoking and HS severity. When we compared clinical and ultrasonographic assessment across the different phenotypes, the results showed that ultrasound is a valuable tool in the evaluation of all observed phenotypes. In the regular and mixed phenotypes, all clinical scoring systems correlated well with ultrasound-based ones. In the follicular and anogenital phenotypes, we found no correlation between Hurley and SOS-HS, but IHS4 and HS-PGA strongly correlated with US-based systems. In the regular phenotype, significant differences were found between all clinical and ultrasound scoring systems. For the follicular phenotype, a significant difference was noted between Hurley and SOS-HS. In the mixed phenotype, differences were observed between Hurley and SOS-HS, and between US IHS4 and IHS4. The presence of correlations suggest that both clinical and ultrasound scoring systems are assessing the same disease parameters, while the lack of correlation may reflect differences in sensitivity, with one method detecting features of the disease missed by the other. The observed differences across the phenotypes underscore the added value of ultrasonography in capturing subclinical and deeper lesions not visible through clinical assessments.
Overall, our results indicate that clinical IHS4 and HS-PGA, alongside their corresponding ultrasound scales (US IHS4 and US HS-PGA), provide more accurate and comprehensive disease staging than the traditional Hurley and SOS-HS staging systems, which are static and less detailed in assessing disease severity. However, ultrasound should complement clinical assessments as both clinical and ultrasound evaluations should go hand in hand to offer a more complete understanding of disease severity.
Limitations. The limitations of the study include the small sample size, being conducted at a single centre, and the absence of a national HS register in Bulgaria. The patients from specialized centres may not be representative of the wider HS population as they might have more severe or even atypical cases.
Conclusions
Hidradenitis suppurativa is a condition that remains underestimated mainly due to delayed diagnosis and atypical presentations. Determining the stage and phenotype of a disease is essential for selecting the most effective treatment strategy. High-frequency ultrasound emerges as an important diagnostic tool for assessing disease severity in patients and provides sensitive information by detecting subclinical lesions. The combination of clinical with ultrasound assessment specifically using the clinical IHS4, HS-PGA along with equivalent ultrasound-based US IHS4 and US HS-PGA staging systems provides the most accurate staging. A multidisciplinary approach in HS Expert Centres may enhance the diagnosis and treatment outcomes in patients, considering the severity of the condition and its associated comorbidities. Prospective future directions of this study include examining the role of ultrasound in treatment applications, with a particular emphasis on its utility in preoperative mapping and the monitoring treatment over time.