Introduction
Human papillomaviruses (HPVs) are DNA non-enveloped viruses affecting the skin and mucosa. Mainly HPV infections are subclinical, without any visible symptoms, and last no longer than 2 years. They are subdivided into two groups: low-risk (LR-HPV, e.g. HPV 1, 2, 6, 8, 11, 34, 40, 42, 43, 44, 61, 69, 71, 72, 81, 83, and 84), commonly associated with benign manifestations (warts, papillomas, condylomata and focal epithelial hyperplasia), and high-risk types (HR-HPV, e.g. HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) associated with malignant manifestations. They are mostly known as the origin of cervical cancer as well as penile, anal and oropharyngeal cancers [1, 2].
HPVs comprise double-stranded circular DNA of approximately 8,000 bp. HPV genome can be divided into 3 functional parts: late (L), early (E), and noncoding long control region (LCR). L region encodes two capsid proteins (L1 and L2) whereas E region encodes regulatory proteins (usually 6 proteins: E1, E2, E4, E5, E6 and E7). One of the key events of HPV-induced carcinogenesis is the integration of the HPV genome into a host chromosome. HPV genome usually resides as nuclear circular plasmid. Integration of the disrupted viral genome into the host genome up-regulates the E6 and E7 expression due to loss of repressor E2 production. The E6 and E7 as viral oncoproteins are crucial in the mechanism of oncogenesis. The HPV E6 and E7 inactivate the p53 and pRB tumour suppressors, respectively. Apoptosis, differentiation, and senescence are combined with p53 and pRB action, disrupted activity leads to cellular immortalization. HR-HPV E6 and E7 proteins can also induce genomic instability, characteristic of human carcinogenesis. LR-HPV oncoproteins are less efficient than HR-HPV oncoproteins in the processes described above [3, 4].
Some data from the last 30 years suggest the association between HPV infections and premalignant lesions (leukoplakia, proliferative verrucous leukoplakia, lichen planus, submucous fibrosis). The relation may be quite logical due to the pathway of the transformation of premalignant lesions to carcinoma, when HPV aetiology of some carcinomas is well known.
Aim
The aim of this paper is to review the current literature to estimate the prevalence of HPV (HPV DNA) detected in samples of oral leukoplakia (OLK).
Material and methods
This systematic review was carried out in accordance with the standards by the PRISMA statement.
A medical literature review was carried out for PubMed/Medline, Scopus and Cochrane Library search engine using the MeSH terms and other key words. We limited our search to studies published in the past 6 years (1 January 2015 – 19 March 2021) and the language of the articles (English). The titles of the articles and abstracts were reviewed. Duplicates and repeated publications were rejected. The full texts of the selected studies were retrieved and further analysed. The following terms were used: oral OR mouth AND leukoplakia OR premalignant OR precancerous OR dysplasia OR potentially malignant disorders OR premalignant lesions AND hpv OR papillomavirus OR papillomaviruses OR papilloma virus.
Eligibility criteria were the following: inclusion criteria: 1. original studies on HPVs in oral leukoplakia, 2. ex vivo studies, 3. studies in the English language, 4. all techniques of material obtaining, and 5. HPV detection based on DNA detection. Exclusion criteria were as follows: 1. studies not done in OLK, 2. studies not done in HPVs, 3. HPV detection based only on p16 immunohistochemical assay, 4. plasma and saliva samples, and 5. no histopathologically confirmed OLK.
For the analysis, we selected all studies evaluating the association between HPV infection and the occurrence of OLK. Full articles were analysed. Types of the study were taken into account (relation of HPV infection and lesion occurrence), the studies were subdivided into 3 types: cohort studies, case-control studies and cross-sectional studies. Identification of titles and abstracts of studies, data extraction were performed independently by two researchers (D.R. and A.B.). Cases of disagreement were resolved by consensus. Data were abstracted. They are presented in Table 1.
Table 1
Leukoplakia
| 1st author | Year | Type of study (relation of HPV infection and lesion occurrence) | Sample size | Type of sample | Method of HPV detection | HPV type detection | Results | |||
|---|---|---|---|---|---|---|---|---|---|---|
| OLK, positive cases –n HPV/n OLK | HPV types detected – type (number of cases) | Control, positive cases –n HPV/n PCHM | HPV types detected – type (number of cases) | |||||||
| Bhargava A | 2016 | Cross-sectional | 50 OLK (0 no dysplasia, 29 mild dysplasia, 20 severe dysplasia, 1 unspecified) | Incisional biopsy or surgical excision, paraffin embedded | qPCR | 16, 18 | 0/50 | – | – | – |
| Chen XJ | 2016 | Cross-sectional | 53 OLK, 6 OLP, 40 OSCC | Incisional biopsy, frozen at –80°C | qPCR, DNA sequencing | 16, 18 | 0/53 | – | – | – |
| Ferreira LL | 2017 | Case-control | 32 OLK (26 no dysplasia, 6 dysplasia), 24 PCHM | Incisional biopsy, frozen at –80°C | nPCR | 6/11/16/ 18/31/33 | 22/32 | HPV X (no restriction patterns analysis) | 11/24 | HPV X |
| Pierangeli A | 2016 | Case-control | 9 OLK, 12 OLP, 24 papillomatosis, 17 other lesions*, 54 PCHM | Oral brush biopsy | qPCR | 6, 11, 16, 18, 31, 33, 53, 58 | 3/9 | HPV 16 (2), 18 (1) | 19/54 | HPV 6 (7), 16 (9), 18 (1), 33 (1), 53 (1) |
| Ramya AS | 2017 | Case-control | 15 OLK, 25 PCHM (10 and 15 individuals – controls without and with deleterious habits) | Incisional biopsy or surgical excision | PCR-RFLP | N | 3/15 | – | 1/25 | – |
| Rebolledo-Cobos M | 2020 | Cross-sectional | 4 OLK, 8 AC, 22 HP, 8 OP, 3 NP, 3 OSCC | Incisional biopsy or surgical excision, paraffin embedded | PCR | 16, 18, 31, 45 | 1/4 | HPV 16 (1) | – | – |
| Saghrava- nian N | 2015 | Case-control | 20 OLK, 114 OSCC, 21 VC, 18 PCHM ** | Incisional biopsy or surgical excision, paraffin embedded | PCR, DNA sequencing | 6, 11, 16, 18, 31 | 0/20 | – | 0/18 | – |
| Sivakumar N | 2021 | Cross-sectional | 25 OLK (6 no dysplasia, 13 mild dysplasia, 4 moderate dysplasia, 2 severe dysplasia), 26 OSCC, 12 OPSCC | Exfoliative brush cytology, frozen at –80°C | PCR | 16 | 5/25 | HPV 16 (5) | – | – |
| Sundberg J | 2019 | Cross-sectional | 74 OLK, 16 OSCC | Incisional biopsy or surgical excision, paraffin embedded | qPCR, p16 | 6, 11, 16, 18, 31, 33, 35, 39, 45, 52, 56, 58, 59 (13 types) | 0/74 | – | – | – |
| Sundberg J | 2020 | Cross-sectional | 432 OLK (236 no dysplasia, 196 dysplasia) | Incisional biopsy or surgical excision, paraffin embedded | qPCR | 6,11,16,18,31,33,35,39,45,52,56,58, 59 (13 types) | 5/432 | HPV 11 (N), 16 (N), 31 (N), 33 (N) | – | – |
| Yang LQ | 2019 | Case-control | 103 OLK (0 no dysplasia, 56 mild dysplasia, 24 moderate dysplasia, 23 severe dysplasia), 30 OSCC, 30 PCHM | Oral brush biopsy | PCR and genotyping by flow-through hybridization | 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, 6, 11, 26, 34, 40, 42, 43, 44, 54, 55, 57, 61, 67, 69, 70, 71, 72, 81, 83, 84 (37 types) | 5/103 | HPV 18 (1), 35 (1), 39 (1), 40 (1), 51 (1), 82 (1) | 1/30 | HPV 68 (1) |
| della Vella F | 2019 | Cross-sectional | 65 OLK (44 without dysplasia, 21 dysplasia) | Oral brush biopsy and incisional biopsy, paraffin embedded | qPCR | 6, 8, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 66, 68, 69, 70, and 73 (28 types) | both methods: 11/65 | HPV 6 (8), 11 (2), 16 (2), 35 (1), 42 (3), 43 (1), 53 (1) | – | – |
| Zendeli-Bedjeti L | 2017 | Case-control | 40 OPML (4 OLK, 1 OEK, 4 AK, 31 OLP), 40 PCHM | Exfoliative brush cytology | qPCR | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59 (12 types) | 4/4 | HPV 16 (2), 18 (2) | 1/40 | HPV 31 (1) |
AC – acanthosis, AK – actinic keratosis, HP – epithelial hyperplasia, HPV X – HPV not distinguishable, HR-HPV – high-risk HPV, ISH – in situ hybridization, LR-HPV – low-risk HPV, N – data not available, NP – nicotine palatinus, nPCR – nested PCR, OEK – oral erythroplakia, OLK – oral leukoplakia, OLP – oral lichen planus, OP – oral papilloma, OPML – oral premalignant lesions, OPSCC – oropharyngeal squamous cell carcinoma, OSCC – oral squamous cell carcinoma, p16-IHC – p16 immunohistochemistry staining, PCHM – patient with clinically healthy oral mucosa, PCR – polymerase chain reaction, PCR-RFLP – PCR and subsequent restriction fragments length polymorphism analysis, VC – verrucous carcinoma, / – It means HPV types are not distinguishable in the following HPV detection method,
The risk of bias was independently examined by two authors (D.R. and A.B.). We did not perform any assessment for every individual study. Cases of disagreement were resolved by consensus of all authors. We did not exclude studies on the basis of risk of bias or low quality evidence.
Results
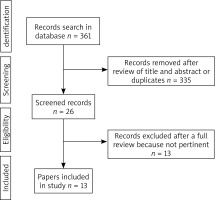
The selection of articles is shown in the flowchart (Figure 1). After reviewing their titles and abstracts and rejecting duplicates, we identified 26 manuscripts to further selection. Among these 26 manuscripts, 13 were excluded due to not pertinent full text (11 studies with no OLK subdivision in a study group or no subdivided outcomes, even the number of OLK cases was known, 2 studies with no OLK). There were 6 articles with case-control studies [5–10], 7 articles with cross-sectional studies [11–17] and no articles with cohort studies.
Firstly, every single study of HPV prevalence was counted separately and tabulated by study type (Table 2). HPV positive cases in OLK ranged from 0% to 100%. Subsequently, studies were tabulated and analysed in two groups (cross-sectional and case-control studies). Each of two groups was evaluated in 4 ways: for HPV 16, HR-HPV, LR-HPV and all HPV prevalence. HPV 16 as the most significant of carcinogenic HPVs was described separately. HPV 16 was also counted in HR-HPV groups. Articles with no distinguishable HPV types or no distinguishable risk group of HPV, with respect to some evaluation (HPV 16, HR-HPV, LR-HPV groups) were rejected and not taken into account. On the contrary, in the last group (all HPV groups) every single case was evaluated. Outcomes are presented in Tables 3 and 4.
In all studies, after rejecting control specimens, overall prevalence of HPV in OLK was 6.66% (59 HPV positive cases out of 886 cases evaluated). Overall HPV prevalence was defined as positive lesions for any oral HPV type, divided by the total population of lesions tested for HPV. Focusing on HPV type detection, detection of HPV 16 was evaluated in all 13 studies, but only in 10 articles HPV 16 was distinguishable and data were given. The prevalence of HPV 16 positive cases was 2.95% (12 of 407). HR-HPVs were evaluated in all 13 articles, but only in 10 articles HR-HPVs were distinguishable and data were given, the prevalence was 5.16% (21 of 407). LR-HPVs were evaluated and distinguishable in 5 of 13 studies, the prevalence was 3.32% (9 of 271). HR-HPVs were detectable in all 13 studies, whereas LR-HPV in 8 studies, which included 84.65% of individuals (745 cases out of 886 cases evaluated).
Table 2
Prevalence of HPV in each study
Table 3
HPV in OLK, cross-sectional studies
Table 4
HPV in OLK, case-control studies
Dysplasia in OLK and HPV infection was analysed as well. We found articles with dysplasia subdivision among all articles. Subsequently, outcomes were analysed to estimate overall prevalence in the group without and with dysplasia. Dysplasia of leukoplakia was mentioned in 7 articles [5, 7, 9, 11, 14, 16, 17]. Four articles were excluded due to lack of HPV detected in some study or not-subdivided outcomes. Three studies were taken into account (Table 5). HPV was detected in 19.56% (9 HPV positive cases of 46 cases evaluated) of lesions with dysplasia, compared to 38.16% (29 of 76) among non-dysplastic lesions. Overall prevalence of HPV in those studies was 31.15% (38 of 122).
Table 5
Prevalence of HPV in OLK without and with dysplasia
There were limitations of some analysed studies associated with HPV-DNA detection: high risk of false outcomes due to non-quantitative PCR and PCR-product visualization on gel [5, 7, 8, 13, 14], and a very small group of OLK [6, 10, 13]. Due to the heterogeneity in the data presentation, a more relevant statistical analysis of these results was not possible, and the results were presented descriptively only with an estimation of the prevalence of HPV.
Discussion
Oral leukoplakia is a lesion in which oral cancer is more likely to occur than in its normal counterpart and is the most common premalignant lesion of the oral mucosa. WHO definition of the lesion is: “Leukoplakia is a clinical term used to describe white plaques of questionable risk, once other specific conditions and other oral premalignant lesions (OPML), have been ruled out” [18]. In differential diagnosis mainly the following diseases are taken into account: candidiasis, chemical burn (e.g. aspirin burn), leukoedema, lichen planus, lichenoid lesion, lupus erythematosus, morsicatio buccarum, psoriasis, white sponge nevus, hairy leukoplakia, keratotic lesion, and geographic tongue [19]. Leukoplakia is a clinical term, but it is typically modified based on histopathological examination [20].
The aetiology of oral leukoplakia is unknown, it seems to be multifocal [21]. There are several risk factors. The main one is the use of tobacco in either smoked or smokeless form. Other risk factors are: the use of areca (betel) nuts, chronic candidiasis, lack of fresh fruits and vegetables in diet, and alcohol consumption [21, 22]. HPV infection is also considered as a risk factor for OLK [1].
HPV genome encodes several regulatory proteins, two of them are oncoproteins (E6 and E7). After the integration of the HPV genome into a host chromosome, which is the key event of HPV-induced carcinogenesis, the host genome up-regulates the E6 and E7 expression. The HPV E6 and E7 inactivate the p53 and pRB tumour supressor, it leads to cellular immortalization and proliferation [3]. In addition, HPV can evade the innate immune system, delaying the adaptive immune response; infected basal cells during turnover are pushed out towards the epithelial surface, avoiding the circulating immune system, which can promote a persistent HPV infection [23].
The association between HPV infection and genital premalignant as well as malignant lesions has been well established, with the evident HPV aetiology [24]. Despite this, the association between oral (not oropharyngeal) squamous cell carcinoma (OSCC) development and HPV infection as an aetiology factor is still under debate [14, 25]. In oral premalignant lesions and in OSCC, it is always presented as a possible relation and it is still not well known [25, 26]. In addition, OPMLs very often are analysed as one lesion, even in WHO Classification of Head and Neck Tumours 2017 as well as in 11 articles of the 26 articles found to this paper, whereas they are different diseases with different aetiology or with unknown aetiology and dissimilar pathogenesis [18], which can lead to misunderstanding and can diminish the impact of the studies.
HPV in OLK is more frequent than one decade ago [26]. Generally, studies are focused on high-risk HPV and HPV 16 detection. HR-HPVs, with more oncogenic nature than self-limiting hyper-proliferative type [4], were more often seen in OLK than LR-HPVs. On the other hand, HPV prevalence in non-dysplastic and dysplastic OLK samples is striking, because it was lower in dysplastic OLK than in non-dysplastic OLK. Nevertheless, the number of cases in those 3 studies is very minimal.
The analysis showed that there might be some slight correlation between HPV and OLK occurrence, but evidence was insufficient. We do not know if or how HPV infection affects leukoplakia growth and dysplasia development, whether HPV can initiate leukoplakia occurrence or leukoplakia lesion favours HPV infection and its persistence.
There are some limitations of our analysis. First of all, the number of reviewed articles is minimal. It could be more, but we rejected 11 reviewed studies due to lack of OLK subdivision, only 13 were analysed. Also the quality of remaining studies leaves a lot to be desired. Population of some study groups was very small. Also, methods of HPV detection could be questionable in some cases. Non-quantitative PCR as well as PCR product visualization on gel are easy to undermine.
Conclusions
We propose extending further studies also to include low-risk HPVs, with more low-risk types, and compare their prevalence with the presence of dysplasia in leukoplakia, especially in case-control studies. Also further studies with the attempt of determining HPV infection impact on leukoplakia are required, both epidemiological and molecular studies. Besides, leukoplakia and other premalignant lesions can no longer be treated as one lesion.