Introduction
Chemotherapy faces substantial challenges in addressing cancer patients because of the resistance displayed by cancer cells to multiple medications. The ineffectiveness of anticancer drugs in eradicating cancer cells stems from a range of factors encompassing disparities in drug absorption, metabolism, and efficient delivery to the intended target tissues. The ineffective nature of chemotherapy is influenced by the challenging penetration of drugs into certain body regions where tumors are located. Additionally, chemotherapy resistance is heightened by the actions of Glutathione S-transferases (GSTs), which decrease the efficacy of chemotherapeutic drugs by detoxifying them within cells [1]. Resistance to chemotherapy is a complex phenomenon influenced by various factors. Several research investigations have repeatedly showcased a correlation between the emergence of multidrug resistance (MDR) and the synchronized expression of efflux transporter proteins alongside GSTs within tumor cells. The intricate interplay between efflux transporter proteins and GSTs is pivotal in the complex processes leading to drug resistance in cancer. Elevated expression of both efflux proteins and GSTs within tumors has the potential to markedly diminish the effectiveness of various anticancer drugs, presenting a formidable challenge in the field of cancer treatment [2,3].
Several alkylating agents integral to contemporary cancer therapy are recognized as substrates for GSTs [4]. Available evidence unequivocally establishes a link between the heightened expression of GSTs’ increased levels of glutathione (GSH) within tumors and their correlation with the escalated manifestation of MDR [5].
Chemotherapy is frequently extensively employed in the treatment of breast cancer. Nevertheless, a recurring obstacle stems from the widespread resistance to chemotherapeutic agents frequently associated with the mechanisms connected to MDR in cancer cells [1].
MDR in human cancer cells may result in heightened drug efflux, a phenomenon facilitated by transporter proteins such as MDR1 (P-glycoprotein) and MRP1. These proteins belong to the transporter proteins found in the ATP-binding cassette (ABC) family [6]. Unlike P-glycoprotein, MRP1 has the capability to function as a GS-X pump. Specifically, it can transport drugs that are conjugated with GSH. Supporting this assertion is evidence suggesting that the rates of ATP-dependent transport of diverse GSH-conjugated compounds correlate with the expression levels of MRP1 in numerous cell lines [7]. ABC transport proteins play a crucial role in expelling GSH conjugates from cells. Despite the potential toxicity associated with specific GSH conjugates, these transporters contribute to drug resistance, similarly to the drug transporter MDR1. Cole et al. [8] established that the MRP1, an efflux transporter for GSH conjugates, plays a role in conferring resistance to a range of compounds that also serve as substrates for MDR1.
The involvement of MRP1 in cisplatin resistance remains a topic of debate. It is worth noting that members of the MRP family exhibit broad and overlapping substrate specificities [9]. While drugs traditionally associated with the “multidrug resistant phenotype” may not be immediately considered for GSH conjugation, some of them could potentially be co-transported with GSH by MRP [10]. The involvement of these MRPs in breast cancer has not undergone comprehensive exploration. Nevertheless, conceivable markers for the ailment involve the expression levels of the MRP breast cancer resistance proteins GST and P-glycoprotein (P-gp). This shows promise and carries significant implications for prognosis.
Aim of the work
The objective of this investigation was to analyze protein expressions of MDR-1 (P-gp), MRP-1, MRP-2, MRP-3, MRP-7, BXP-34, and BXP-21 within the ABC transporter protein families, and GST protein expressions (GSTA1, GSTK1, GSTM1, GSTO1, GSTP1, GSTS1, GSTT1, GSTZ1), investigated in 50 neoadjuvant and 95 adjuvant breast cancer patients. Statistical analyses were conducted to examine the expression differences of these markers between the two groups. Additionally, correlations were established between the expression levels and the clinical information of the patients.
Material and methods
Patients
Tumor and surrounding tumor free breast tissues of 145 invasive ductal breast cancer patients were received from the Dr. Abdurrahman Yurtaslan Ankara Onkology Research and Education Hospital. Among 145 patients, 50 received preoperative (neoadjuvant) chemotherapy, and 95 received postoperative (adjuvant) chemotherapy. Therefore, the patients were divided into two groups: 50 neoadjuvant breast cancer patients and 95 adjuvant breast cancer patients. Neoadjuvant breast cancer patients had platinum- based chemotherapy before surgery. The clinical data of the patients is shown in Table 1.
Table 1
Clinical data of neoadjuvant and adjuvant patient groups
Immunohistochemical procedure
For immunohistochemical staining, 145 formalin-fixed, paraffin-embedded tissues sections, after deparaffinization, were incubated with 3% hydrogen peroxide for 10 minutes. The sections were boiled in a pressure cooker with a citrate buffer of pH 6.0 for 3 minutes. The sections were then incubated for 10 minutes at room temperature with protein blocking (SHP125; Scy Tek laboratories, West Logan, UT). Sections were incubated with diluted primary antibodies (1:750 for GSTP1 Boster (PA1590), 1:400 for GSTM1Santa Cruz (1H4F2), 1:350 for GSTT Bioss (bs-13400r), 1:250 for GSTA1 Bioss (bs-13398R), 1:250 for GSTS1 Santa Cruz (SC-30067), 1:500 for GSTZ1 Bioss (bs-13442R), 1:300 for GSTO1 Bioss (bs-5160R), 1:500 for GSTK1 Bioss (bs- 13399R), 1:100 for MDR Bioss (bs-0563R), 1:250 for MRP1Boster (PA1634), 1:250 for MRP2 Bioss (bs-1092R), 1:250 for MRP3 Bioss (bs-0656R), 1:250 for MRP7 Abcam (ab130460), 1:100 for BXP21 Santa Cruz, 1:150 for BXP 34, Abcam (ab3379)) for 1 hour. The secondary antibody streptavidin-proxidase complex (SHP 125) (ScyTek Laboratories, West Logan, UT, USA) was applied for 10 minutes. Diaminobenzidine (DAB) was then incubated to monitor peroxidase activity. Hematoxylin was used for counterstaining. Tissue sections were evaluated by two expert pathologists. Immunohistochemical assessments were conducted based on the staining intensities observed in the tissues under a light microscope. The scoring system included (0) for negative staining (indicating no protein expression), (+1) for weak staining, (+2) for moderate staining (reflecting a moderate level of protein expression), and (+3) for strong staining (indicating a strong level of protein expression) [11].
Statistical analysis
The study employed MINITAB 14 statistical software (MINITAB® release 14.12.0. MINITAB INC, State College, Pennsylvania, United States) for statistical evaluations. Expression differences were scrutinized through the Pearson correlation, while the relationships between clinical data were investigated using the Mann-Whitney U-test.
Results
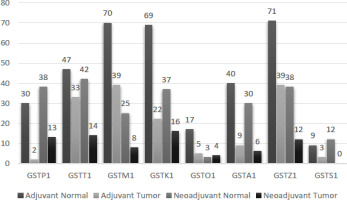
A total of 145 patients were included in the study, with 50 undergoing neoadjuvant breast cancer treatment, and 95 receiving adjuvant breast cancer therapy. The analysis of breast cancer tissues revealed a notably elevated expression of all studied GST proteins in comparison to the normal breast epithelial cells of patients who underwent adjuvant treatment (Tables 2, 3). The data in Table 2 indicates a statistically significant increase in the expressions of GSTP1, GSTM1, GSTA1, GSTZ1, and GSTK1 in tumor tissues compared to normal tissues (p<0.05) (Figures 1, 2).
Table 2
Expressions of GSTs in tumor and normal tissues of adjuvant breast cancer
[i] Notes: The staining scores were calculated based on the sum of the staining intensity of positively stained tumor and normal tissues. Staining intensity was graded as: 0 for no staining, 1 for weak, 2 for moderate and 3 for strong staining. Differences of GSTs and MDR protein expressions between tumor tissues of neoadjuvant and adjuvant breast cancer patients were examined by the Mann-Whitney U test with 95% confidence level. The number of neoadjuvant patients was 95. *T/N – rate of tumor of neoadjuvant breast cancer patients and tumor of adjuvant breast cancer patients. ** – p-value less than 0.05 was considered statistically significant.
Table 3
Expressions of GSTs in tumor and normal tissues of neoadjuvant breast cancer patients
[i] Notes: The staining scores were calculated based on the sum of the staining intensity of positively stained tumor and normal tissues. Staining intensity was graded as: 0 for no staining, 1 for weak, 2 for moderate and 3 for strong staining. Differences of GST expressions between tumor and normal tissues were examined by the Mann-Whitney U test with 95% confidence level. The number of neoadjuvant patients was 50. *T/N – rate of tumor and normal. ** – p-value less than 0.05 was considered statistically significant.
Figure 1
Immunohistochemical expressions of GST proteins in patients with breast cancer
Notes: (a) Expression of GSTP1 protein in breast carcinoma tissue, x100; (b) GSTP1 negative staining in normal breast tissue, x200; (c) Expression of GSTT1 protein in breast carcinoma tissue, x200; (d) GSTT1 negative staining in normal breast tissue, x100.
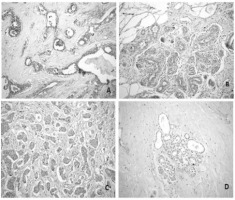
Figure 2
Immunohistochemical expressions of GST proteins in patients with breast cancer
Notes: (a) Expression of GSTM1 protein in breast carcinoma tissue x100; (b) GSTM1 weak staining in breast normal tissue, x200; (c) Expression of GSTK1 protein in breast carcinoma tissue, x200; (d) GSTK1 weak staining in breast normal tissue, x200.
The protein levels of GSTP1, GSTM1, GSTT1, GSTA1, GSTK1, GSTZ1, and GSTS1 displayed a significant increase in neoadjuvant breast cancer patient tumors compared to the surrounding tumor-free (normal) tissue (p<0.05). Conversely, there were no statistical differences in GSTO1 protein expression between breast tumors and normal tissue (p>0.05) (Tables 2, 3).
When the expression differences of GST and drug resistance proteins in the tumor tissues of patients receiving neoadjuvant and adjuvant treatment were compared, it was found significant that the expressions of GSTP1 and GSTT1 isoenzymes in the tumor tissues of patients receiving neoadjuvant treatment were higher than in the tumor tissues of patients receiving adjuvant treatment (p<0.05). Moreover, it was found significant that MRP3, one of the drug resistance proteins, was similarly high in those receiving neoadjuvant therapy (p<0.05) (Table 4).
Table 4
Expressions of GSTs and MDR proteins in tumor tissues of neoadjuvant and adjuvant breast cancer patients
[i] Notes: The staining scores were calculated based on the sum of the staining intensity of positively stained tumor and normal tissues. Staining intensity was graded as: 0 for no staining, 1 for weak, 2 for moderate and 3 for strong staining. Differences of GSTs and MDR protein expressions between tumor tissues of neoadjuvant and adjuvant breast cancer patients were examined by the Mann-Whitney U test with 95% confidence level. The number of neoadjuvant patients was 95, the number of adjuvant patients was 50. *T/N – rate of tumor of neoadjuvant breast cancer patients and tumor of adjuvant breast cancer patients. ** – p-value less than 0.05 was considered statistically significant.
The protein expressions of MDR proteins in both tumor and normal tissues were investigated in adjuvant and neoadjuvant breast cancer patients (Tables 5, 6).
Table 5
Protein expressions of MDR proteins in tumor and normal tissues with neoadjuvant breast cancer patients
[i] Notes: The staining scores were calculated based on the sum of the staining intensity of positively stained tumor and normal tissues. Staining intensity was graded as: 0 for no, 1 for weak, 2 for moderate and 3 for strong staining. Differences of MDR proteins expression between tumor and normal tissues were examined by the Mann-Whitney U test with 95% confidence level. *T/N – rate of tumor and normal. ** – p-value less than 0.05 was considered statistically significant.
Table 6
Protein expressions of MDR proteins in tumor and normal tissues with adjuvant breast cancer patients
[i] Notes: The staining scores were calculated based on the sum of the staining intensity of positively stained tumor and normal tissues. Staining intensity was graded as: 0 for no, 1 for weak, 2 for moderate and 3 for strong staining. Differences of MDR proteins expression between tumor and normal tissues were examined by the Mann-Whitney U test with 95% confidence level. *T/N – rate of tumor and normal. ** – p-value less than 0.05 was considered statistically significant.
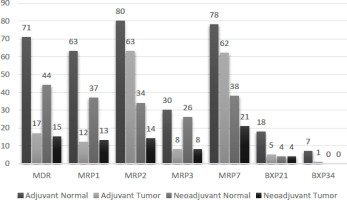
In adjuvant tumors, there was a notable increase (p<0.05) observed in the protein concentrations of MDR1, MRP1, MRP2, and MRP3 when compared to the adjacent normal tissue. In contrast, there were no statistical differences in the levels of MRP7, BXP21, and BXP34 expressions between adjuvant tumor and normal tissues (p>0.05) (Tables 5,6).
In neoadjuvant tumors, the protein levels of MDR1, MRP1, MRP2, MRP3, and MRP7 demonstrated a substantial increase compared to their corresponding normal tissue counterparts (p<0.05) (Figure 3). Nevertheless, there were no statistically significant distinctions in BXP21 and BXP34 expression between neoadjuvant tumors and normal tissue (p>0.05) (Tables 5,6).
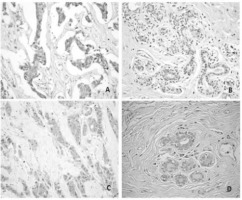
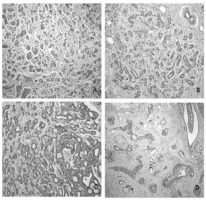
Figure 3
Immunohistochemical expressions of MRP3, MDR1 and MRP1 in patients with breast cancer
Notes: (a) Expression of MRP3 protein in breast cancer tissue, x100; (b) MRP3 weak staining in breast normal tissue, x100; (c) Expression of MDR1 protein in breast cancer tissue, x200; (d) MRP1 staining in breast cancer tissue, x100.
Tables 2 and 3 present the protein expressions of tumor tissues from adjuvant and neoadjuvant breast cancer patients, along with their corresponding statistical variances. Notably, tumor tissues from neoadjuvant patients exhibited significantly higher expressions of GSTP1, GSTT1, and MRP3 compared to tumor tissues from adjuvant patients (p<0.05). Conversely, there were no statistically significant differences observed in the expressions of GSTM1, GSTA1, GSTK1, GSTZ1, GSTO1, GSTS1, MDR1, MRP1, MRP2, MRP7, BXP21, and BXP34 between neoadjuvant and adjuvant breast cancer tissues (p>0.05) (Table 4).
There were notable positive correlations between the patients’ smoking status and the protein expressions of GSTO1 (r=0.332; p=0.001), GSTM1 (r=0.222; p=0.031), MRP3 (r=0.295; p=0.004), MDR1 (r=0.198; p=0.054), and BXP34 (r=0.200; p=0.052) in individuals treated with adjuvant therapy. Higher expressions of GSTO1, GSTM1, MRP3, MDR1, and BXP34 in smokers were found to be statistically significant than that of the controls (p<0.05).
In patients undergoing adjuvant therapy, a statistically significant positive correlation (r=0.291; p=0.004) was observed between GSTO1 protein expression and tumor grade. Similarly, there was a statistically positive correlation between GSTO1 (r=0.305; p=0.003) and GSTT (r=0.211; p=0.04), MRP3 (r=0.248; p=0.015), and MRP1 (r=0.288; p=0.053) expressions, as well as the tumor stage in adjuvant patients. Higher expression of GSTO1 and GSTT proteins in poorly diagnosed breast cancer patients was found to be statistically significant (p<005).
Patients treated with adjuvant therapy exhibited a statistically significant negative correlation (r=-0.210; p=0.041) between GSTP1 protein expression and their progesterone status. In contrast, a positive correlation was observed between GSTM1 (r=0.226; p=0.028) expression and the c-erb-2 status in patients treated with adjuvant therapy. Furthermore, a positive correlation was identified between GSTP1 (r=0.342; p=0.015) expression and c-erb-2 status in patients treated with neoadjuvant therapy (p<0.05).
A significant positive correlation (r=0.323; p=0.022) was found between the expression of MRP7 and the progesterone status in individuals undergoing neoadjuvant treatment. Furthermore, a positive association was found between MRP7 (r=0.44; p=0.001) expression and estrogen receptor status in neoadjuvant- treated individuals (p<0.05). Conversely, a negative correlation emerged between MRP7 (r=-0.47; p=0.001) expression and c-erb-2 status in those undergoing neoadjuvant treatment.
Expression of GST isoenzymes in breast cancer patients receiving neoadjuvant and adjuvant therapy is presented in Figures 4 and 5.
Discussion
In this research, the primary aim was to explore the potential link between GST expression in breast cancer and resistance to chemotherapy. It is noteworthy that this study represents the first comprehensive endeavor to provide a detailed representation of the expression patterns of all GSTs in both breast tumors and corresponding control tissues.
In the current investigation, an examination of GST expressions was conducted through immunohistochemistry in 95 cases of adjuvant breast cancers and 50 cases of neoadjuvant breast cancers. The study distinctly demonstrates the immunoreactivity of GSTs, detected through GSTP1, GSTT1, GSTM1, GSTK1, GSTO1, GSTA1 and GSTZ1, and which can be observed when comparing adjuvant breast cancers to neoadjuvant breast cancers. Immunostaining exhibited heterogeneity and notably manifested in epithelial cells (Figure 1).
The group of GST enzymes has been linked to resistance against chemotherapeutic drugs for a prolonged period. Overcoming chemoresistance remains a considerable challenge in cancer therapy, and the alteration of cellular proteins, such as GSTPs involved in detoxification, has been suggested as one of the mechanisms contributing to the development of drug resistance [12]. In particular, the connection between GST-pi expression and clinical drug resistance has been identified [13]. In line with these discoveries, Su et al. [14] illustrated GSTP immunoreactivity in breast cancer tissues obtained from 42 female patients. The research revealed that the existence of GSTP in breast cancer tissue acts as an adverse prognostic indicator, as tumors with heightened GSTP levels demonstrated considerable resistance to chemotherapy. Therefore, there is an implication that GSTP may play a crucial role in deactivating one or more of the chemotherapeutic agents used in this treatment. In a related study, Huang et al. [15] noted significantly poorer disease-free survival in patients with breast tumors positive for GSTP who received adjuvant chemotherapy post-surgery as opposed to patients with tumors negative for GSTP. Immunohistochemistry has demonstrated the expression of various GSTs, including GSTA1, GSTM1, GSTP1, and GSTT1, in human breast tumors and normal breast tissue [16]. Our results suggest that the average levels of GSTP1 and GSTT1 were increased in breast cancer tissue after treatment, in contrast to the levels observed in normal breast tissue.
Consequently, the increased activity of the GSTs in breast tumors may be associated with the developed resistance of the tumors against anticancer drugs. GSTP1 and GSTT1 play a role in the intrinsic and acquired resistance of tumors to anticancer drugs [17].
In the current investigation, the localization and distribution of MDR expression were examined in 95 adjuvant and 50 neoadjuvant breast cancer patients, as well as the adjacent tumor-free breast tissues employing immunohistochemistry.
This investigation incorporated a semi-quantitative assessment of MDR expression in breast tissues. A parallel methodology has been applied in earlier studies to scrutinize the expression of different enzymes, e.g. aromatase in cases of breast carcinomas [18]. The findings revealed a heightened level of MRP1 expression in tumors (Table 4) among the patients. Another noteworthy observation in the current study is the increased expression of MRP3 in tumors. Additionally, when the tumor tissues of patients who received chemotherapy and those who did not receive chemotherapy were compared, MRP3 was found to be statistically significant. Our results demonstrate that both the quantities and variations in GST proteins are heightened in infiltrating ductal carcinoma tissue when compared to normal breast tissue. In particular, GSTP1 and GSTT1 prominent proteins in normal breast tissue exhibited increased levels in the corresponding cancer tissues of the majority of patients. This suggests that there might have been specific alterations in the regulation of GST expression during and/or after carcinogenesis. Our discovery of the M1 phenotype in the tissues of 40% of patients aligning with the prevalence of the M1-null phenotype in the general population implies that its absence may not inherently increase the risk for this particular tumor type. This is in contrast to observations in other cases, such as lung cancer [19].
In our examination, the expression of multidrug resistance-associated proteins was explored, specifically MRP1, MRP2, MRP3, and MRP7, in samples collected from patients undergoing adjuvant and neoadjuvant therapies for breast cancer. The main objective was to evaluate the potential involvement of these proteins in clinical drug resistance.
The potential involvement of MRP proteins in clinical breast cancer has been investigated in earlier studies, with a specific emphasis on MRP1; however, the results are not entirely conclusive. Some authors report a decrease in MRP1 expression in breast carcinoma with poorly differentiated histology, implying a link between the loss of MRP1 and a lack of differentiation [20]. On the contrary, an association suggesting an increase in MRP1 with tumor progression has also been proposed. These conflicting observations highlight the complexity of MRP1’s role in breast cancer and underscore the need for further research to elucidate its precise involvement in different aspects of tumor behavior [21].
Most members of the MRP family are involved in the translocation or conjugation of various structurally diverse endogenous or xenobiotic compounds. It is noteworthy that some members of the MRP family are specifically characterized by their participation in GSH transport [20].
In a retrospective review carried out by Larkin et al. [22], the study concentrated on the expression of MDR-1 and MRP-1 in 177 instances of invasive breast carcinomas. The results emphasized a robust connection between MDR-1 expression and an elevated histologic grade (grade III). Furthermore, a particularly noteworthy association was detected between the expressions of MDR-1 and MRP-1 (p<0.01) [22].
In a study conducted by Faneyte et al. [23], the results indicated detectable MRP1-3 mRNA in all breast cancer cell lines and tumor samples. Importantly, the researchers observed no increase in expression between untreated carcinoma samples and those obtained after neoadjuvant anthracycline treatment [23].
Keith et al. [24] conducted investigations that exposed heightened mRNA levels of GSTP and MDR1 in initial biopsies of human breast tumors collected before the onset of chemotherapy. These findings held true across tumors exhibiting diverse inherent reactions to doxorubicin treatment, encompassing colon cancer, head and neck squamous cell carcinomas, and myeloid leukemias [24].
Terrier et al. [25] illustrated heightened expression of pi class GST and MDR (P-glycoprotein) genes in both multidrug-resistant MCF-7 cells and toxin-resistant rat hyperplastic hepatic nodules. These findings imply that these genes might have common regulatory mechanisms [25].
The outcomes reported by Maliepard et al. [1] and Scheffer et al. [26], which demonstrate the detection of BCRP protein at noticeable levels in breast tumor tissues using immunohistochemical staining techniques, align with our own immunohistochemical results. This agreement provides additional support for the existence of BCRP protein in breast tumors.
In the research carried out by Faneyte et al. [22], the assessment of BCRP mRNA levels involved real-time reverse transcription-PCR, and immunostaining was executed on 9 cell lines related to breast cancer. The investigation comprised samples from 25 primary breast carcinomas and 27 individuals who underwent preoperative anthracycline-based therapy. The results suggested that there was no notable disparity in BCRP expression between untreated and treated tumor samples. Moreover, BCRP expression exhibited no association with diminished response or survival in the cases scrutinized. This implies that within the context of this study, BCRP may not serve as a substantial predictor of treatment response or overall survival in breast cancer patients undergoing preoperative anthracycline-based therapy.
The current study indicates an elevated expression of GST proteins in both tumor and normal breast tissue, with distinct patterns noted in GSTP1 and GSTT1 in breast tumors. However, further research is needed to fully understand the precise impact of these findings on breast cancer pathogenesis.
Notably, this study is the first to comprehensively detail the tissue-specific expression of GSTs and MDR and MRPs collectively in both adjuvant and neoadjuvant breast cancer patients. Significantly, higher expressions of GSTP, GSTT, and MRP3 were observed in the tumors of neoadjuvant patients compared to adjuvant patients. This observation opens avenues for deeper investigations into the implications of these expression patterns in the context of breast cancer progression and treatment responses.
While survival data for the subjects in the current study is currently unavailable, a notable correlation has been observed between GST expression and well-established prognostic indicators. Particularly, a positive correlation was detected between the expressions of GSTO1, GSTT1, MRP3, and MRP1 and both tumor grade and stage. Instances characterized by higher grades and stages, typically associated with a less favorable prognosis, demonstrated a higher likelihood of expressing these proteins. This robust correlation implies the potential utility of assessing the expression of GSTO1, GSTT1, MRP3, and MRP1 in a tumor as an indicative marker for a poorer prognosis. Additional investigations and survival analyses could yield valuable insights into the prognostic significance of these protein expressions in individuals with breast cancer.
Our observations revealed a positive correlation between the expressions of GSTM1, GSTO1, MRP3, MRP1, and BXP34 and the smoking status of the individuals in the study. The findings specifically highlighted a significant relationship between the expressions of GSTM1 and GSTO1 and the smoking status of breast cancer patients. Recognizing the expression of xenobiotic metabolizing enzymes in tumors is a potentially significant factor influencing anti-tumor drug resistance. This acknowledgment underscores the importance of understanding how these enzymes may impact the metabolism and efficacy of therapeutic agents in cancer treatment. The quantity and relative proportions of various enzymes within tumors contribute to determining resistance to anti-cancer drugs.
Leveraging the increased expression of specific GSTs in diverse cancers allows for the efficient buildup and/or triggering of anti-cancer drugs within cancerous cells. This underscores the suitability of GSTs as biomarkers for combined therapies utilizing distinct GST inhibitors and for the advancement of new anti- cancer medications with targeted precision.
When anticancer agents enter tumor cells, the levels of GSH and the expression of GST enzymes increase within the cell. GSH is a compound that contains a thiol group and is a tripeptide without a protein structure found within the cell. This compound serves as a natural substrate for the GST enzyme. With the help of GSH, the enzyme facilitates the efflux of xenobiotics (for example, anticancer drugs) from the cell through various pumps. The prolonged presence of the drug within the cell becomes more difficult with increased GST activity. Furthermore, an increase in the expression of efflux pumps is observed alongside increased GST activity. Therefore, the high levels of GSH and the overexpression of GST in tumor cells are believed to parallel the development of MDR.
This study suggests that the levels of GSTP1 and GSTT1 isozymes are higher in the neoadjuvant treatment group compared to the adjuvant treatment group, indicating that these isozymes may contribute to chemotherapy resistance. Additionally, MRP-3 from the ABCC superfamily is higher in the neoadjuvant treatment group compared to the adjuvant treatment group, suggesting its potential role in breast cancer development. Moreover, it can be speculated that along with the increased levels of GSTP1 and GSTT1 isozymes during drug efflux, MRP-3, when conjugated with glutathione, reduces the amount of the drug within the cell.