Introduction
Diabetic foot syndrome (DFS) is an infection and/or ulceration and/or destruction of deep tissues of the foot (e.g. bones) occurring in the course of diabetes. The syndrome is caused by damage to the peripheral nerves and/or vessels of the foot, of varying degrees. The resulting ulcers, if not treated properly, can lead to a complication called diabetic foot ulcer syndrome [1, 2].
The International Diabetes Federation (IDF) estimates that DFS, as a serious complication of diabetes, affects 40 to 60 million diabetic patients worldwide (6.3%) [2, 3]. In Poland, according to statistical data, the incidence of DFS is estimated at some 3.5 million patients [1, 2, 4, 5].
The risk of developing DFS increases with age, which phenomenon is closely related to the longer duration of diabetes, cumulative effects of hyperglycaemia and more frequent occurrence of micro- and macro-vascular complications [6–9]. Due to the aetiology, DFS is divided into three types: neuropathic (35%), ischemic (15%), and mixed neuropathic-ischemic (50%) ones [10]. If treatment is delayed, vascular and neuropathic complications lead to DFUs, which may result in gangrene or even foot amputation [11, 12].
The treatment of DFU is difficult and expensive, causing a psychological and financial burden for patients and their families. Due to the complex pathomechanism, the treatment of diabetic foot syndrome should be managed by a multidisciplinary team [13–16].
In addition to pharmacological treatment, patients suffering from DFS can also use various alternative treatment methods, such as hyperbaric oxygen therapy or ozone therapy, which are recommended in the treatment of DFS [16–18].
The use of ozone (O3) within the medical community began in the 19th century. The research conducted so far has confirmed that ozone can increase the efficiency of wound healing by improving skin microcirculation in the case of pressure sores, burn wounds and other ulcers associated with peripheral vascular disease. Currently, increasing evidence has confirmed that O3 can also be used to treat DFS [19–26].
Although O3 therapy with the application of appropriate doses is safe and does not cause side effects, when used outside the “therapeutic window” it may be toxic. It has been proven that at ozone concentrations of 5–10 mg/l and lower there is a therapeutic effect with a large margin of safety for the patient [27–31].
Aim
The aim of the study was to compare the effectiveness of treatment in patients with DFS using local O3 therapy depending on the O3 concentration, based on planimetric assessment of changes in the ulcer and the intensity of accompanying pain.
Material and methods
This was a single-centre, parallel-group study comprising 50 patients in the age range between 39 and 84 years with type 2 diabetes complicated with DFS (neuropathic-ischemic type), treated with the use of local O3 therapy from 2023 to 2024. The patients were divided into two study groups:
The group of patients subjected to a cycle of local O3 therapy using ozone at 30 µg/ml concentration – 25 patients (10 women and 15 men).
The group of patients subjected to a cycle of local O3 therapy using ozone at 60 µg/ml concentration – 25 patients (16 women and 9 men).
Inclusion criteria for the study were as follows: age between 18 and 85 years, diabetic foot ulcer diagnosed in the right or left foot area, ankle-brachial index between 0.7 and 1.2, ulcer surface area > 2 cm2, Wagner grade 2, 3 or 4, patient’s disqualification for endovascular ablation for medical reasons, informed and voluntary consent of the subject to participate in the study.
Exclusion criteria comprised: limb ulcer not associated with diabetic foot syndrome, pregnancy; hypersensitivity to ozone, contraindications to O3 therapy, patients with malignant transformation of diabetic ulcers – skin cancer, patients who did not comply with the treatment or had incomplete clinical data, comorbidities (hypertension, smoking habit), Charcot joint, infectious diseases, critical limb ischemia and/or or previous vascular or orthopaedic procedures, as well as lack of informed and voluntary consent of the subject to participate in the study.
The diagnosis of DFS was based on the diagnostic criteria set out in the International Diabetic Foot Working Group guidelines [4].
The randomization procedure consisted of tossing a coin of PLN 1. This method is widely considered to be the simplest randomization procedure [29]. The obverse of the coin meant assignment to group 1, and the reverse of the coin meant assignment to group 2. Study participants were not informed which group they were assigned to.
Before starting a cycle of local O3 therapy, surgical wound debridement was conducted to remove necrotic tissues or purulent infiltration accompanying the ulcer. Before the study, patients’ baseline clinical and demographic characteristics were also recorded, including height, weight, and duration of diabetes.
Before and after the end of the therapy (10 weeks), a planimetric assessment of the wound surface area was performed. A computer software for planimetric assessment of wound surface area was used for digital image processing [32].
Additionally, before the beginning and after the end of the therapeutic cycle the assessment of pain intensity was performed with the use of VAS scale.
Oxygen ozone therapy procedures
Local O3 therapy with the use of a gaseous oxygen-ozone mixture (5% O3 and 95% O2) with close monitoring of patients was performed once a day for 5 days a week, with a weekend (Saturday–Sunday) break, in two series consisting of 15 treatment procedures each (for a total of 10 weeks). Each treatment lasted 30 min. The intermission between the two series of treatments was 4 weeks, introduced in order to counteract the potential risk of exposing patients to the negative effects of long-term exposure of the skin around the ulcer and deeper tissues to O3.
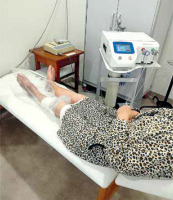
The following treatment procedure was used: patients were a semi-sitting position and wound dressings were removed. The treated limb was placed in a polyethylene bag and sealed (Figure 1). At the end of the treatment, the wound was covered with sterile dressings, in order to maintain proper humidity and improve wound cleanliness. During the procedures, good ventilation of the rooms was ensured so as not to expose patients to additional O3 inhalation.
During the examination, all patients continued their anti-hyperglycaemic therapies. Moreover, during a cycle of physical procedures, in both groups of patients similar conventional pharmacological treatment was applied, including the administration of: sulodexide, micronized purified flavonoid fraction, pentoxifylline, and acetylsalicylic acid in standard doses. Patients from both groups were also treated with insulin therapy to maintain the proper fasting serum glucose level below 6 mmol/l.
Statistical analysis
Statistical analysis was performed with the use of Statistica13 package (StatSoft, Poland). The Shapiro-Wilk test was used to test the normality of data. The distribution of all data was not normal. The Mann Whitney U test and Wilcoxon test were used to compare the two, unmatched and matched, groups of non-parametric data, respectively. Qualitative variables were assessed using the χ2 test. The level of statistical significance was set at p < 0.05. The effect size was calculated, with r = 0.1 indicating a small effect, r = 0.3 indicating a medium effect, and r = 0.5 indicating a large effect. G*power software version 3.1.9.7 (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) (http://www.gpower.hhu.de) was used to determine the power using 2-sided testing, with α = 0.05, and sample size of 25 per group [33]. The outcome used for power analysis was ulcer surface area (cm2). The effect size was 0.72. The power (1-β err prob) was calculated as 0.70. The test family was “t-test” and statistical test was the difference between two independent means (two groups). The type of power analysis was “Post hoc: Compute achieved power – given α, sample size, and effect size”.
Results
The patient characteristics, including the division into study groups, are presented in Table 1.
Table 1
Demographic profile patients of studied groups
| Parameter | Total n (%) | Group 1 n (%) | Group 2 n (%) | *P-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 24 (48) | 15 (60) | 9 (36) | 0.089 |
| Female | 26 (52) | 10 (40) | 16 (64) | |
| Age [years] | ||||
| < 70 | 26 (52) | 13 (52) | 13 (52) | 1 |
| ≥ 70 | 24 (48) | 12 (48) | 12 (48) | |
| Body mass index (BMI) [kg/m2] | ||||
| 18.5–24.99 | 24 (48) | 11 (44) | 13 (52) | 0.571 |
| 25.0–29.99 | 26 (52) | 14 (56) | 12 (48) | |
| Wound ulcer duration [years] | ||||
| < 3 | 22 (44) | 10 (40) | 12 (48) | 0.568 |
| ≥ 3 | 28 (56) | 15 (60) | 13 (52) | |
| Wound ulcer location | ||||
| Left leg | 24 (48) | 11 (44) | 13 (52) | 0.571 |
| Right leg | 26 (52) | 14 (56) | 12 (48) | |
The mean age of all treated patients was 67.1 ±10.6 years, 66.6 ±12.6 years in group 1 and 67.5 ±8.5 years in group 2. The difference in the age of patients between group 1 and group 2 was not statistically significant (p = 0.891). The BMI index was 25.74 ±1.81 kg/m² in group 1 and 24.73 ±2.30 kg/m² in group 2, on the average. Also in this case, the difference between the groups was not statistically significant (p = 0.196). The average duration of diabetes was: 3.12 ±6.4 years in group 1 and 2.89 ±5.48 years in group 2. The difference was not statistically significant (p = 0.231).
Before the beginning of therapy, the mean values of the ulcer surface area in both groups did not differ statistically significantly (p = 0.174) and were: median (Q1–Q3): 7 (7.0–8.0) cm² in group 1 and median (Q1–Q3): 7 (6.1–7.5) cm² in group 2. After the treatment, the average values of the ulcer surface area in group 2 were statistically significantly smaller than in group 1. The median (Q1–Q3) of the ulcer surface area in group 1 was 4.5 (4.0–5.0) cm², and in group 2 it was 4 (3.0–4.5) cm² (p = 0.027), and in both study groups the mean values of the ulcer surface area after the end of treatment were statistically significantly lower as compared to the baseline values before the start of therapy (p < 0.001) in both groups (Table 2).
Table 2
Comparison of mean values of the ulcer surface area between groups 1 and 2 and before and after treatment, along with statistical analysis
| Variable | Ulcer surface area [cm2] | Effect size | **P-value (before vs. after) | |
|---|---|---|---|---|
| Pre-treatment median (Q1–Q3) | Post-treatment median (Q1–Q3) | |||
| Group 1 | 7 (7–8) | 4.5 (4–5) | 0.79 | < 0.001 |
| Group 2 | 7 (6.1–7.5) | 4 (3–4.5) | 0.84 | < 0.001 |
| Effect size | 0.19 | 0.32 | ||
| *P-value (Group 1 vs. Group 2) | 0.174 | 0.027 | ||
Before commencing the therapy, the mean values of pain intensity assessed on the VAS scale in both groups did not differ statistically significantly (p = 0.361) and were as follows: median (Q1–Q3) 8 (7–8) points in group 1 and median (Q1–Q3): 7 (7–8) points in group 2. After treatment, the mean values of pain intensity in group 2 were statistically significantly lower than in group 1. The median (Q1–Q3) of pain intensity in group 1 was 5 (4–5) points, and in group 2 it amounted to 4 (3–4.5) points (p = 0.002), and in both study groups the mean pain intensity values after the end of treatment were statistically significantly lower compared to the baseline values before the start of therapy (p < 0.001) in both groups (Table 3).
Table 3
Comparison of the intensity of pain experienced between groups 1 and 2 and before and after treatment, along with statistical analysis
| Variable | VAS score [points] | Effect size | **P-value (before vs. after) | |
|---|---|---|---|---|
| Pre-treatment median (Q1–Q3) | Post-treatment median (Q1–Q3) | |||
| Group 1 | 8 (7–8) | 5 (4–5) | 0.83 | < 0.001 |
| Group 2 | 7 (7–8) | 4 (3–4.5) | 0.87 | < 0.001 |
| Effect size | 0.14 | 0.4 | ||
| *P-value (Group 1 vs. Group 2) | 0.361 | 0.002 | ||
In both study groups, the percentage changes in the area of ulcers and the intensity of pain after the end of treatment compared to the baseline values were also calculated. In group 1, the mean values of the ulcer surface area after treatment decreased by median (Q1–Q3): 37.50 (28.57–42.86)%, and in group 2 by 44.44 (37.50–51.61)% (p = 0.013). In group 1, the mean pain intensity values assessed using the VAS scale decreased by median (Q1–Q3): 37.50 (28.57–50.00)%, in group 2 by 50.00 (42.86–62.5)% (p = 0.003) (Table 4).
Table 4
Comparison of the percentage of improvement after treatment between groups 1 and 2 in terms of reducing the size of wounds and pain, along with statistical analysis
| Variable | Group 1 | Group 2 | Effect size | *P-value (Gr. 1 vs. Gr. 2) |
|---|---|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | |||
| Percentage change of the wound surface area post-treatment | 37.5 (28.57–42.86) | 44.44 (37.5–51.61) | 0.22 | 0.013 |
| Percentage change of VAS score post-treatment | 37.5 (28.57–50) | 50 (42.86–62.5) | 0.42 | 0.003 |
None of the assessed patients from groups 1 and 2 achieved 100% improvement in the form of complete wound healing. In 3 (12%) patients in group 1, and 9 (36%) patients in group 2, the wound area was reduced by more than 50%. The slightest improvement in the reduction of the ulcer surface area, i.e. 14.28%, was recorded in group 1, and the biggest, i.e. 81.81%, in group 2. In no patient, either in group 1 or 2, was there any increase in the wound surface area after the application of treatment.
No patient in either group 1 or group 2 achieved 100% improvement in the form of complete relief from pain. In 7 (28%) patients in group 1 and 14 (56%) patients in group 2, the intensity of pain experienced was reduced by more than 50%. The least significant improvement in reducing the intensity of pain, i.e. 12.5%, was achieved in group 1, and the most significant, i.e. 71.42% in group 2. In none of the patients, either in group 1 or group 2, the intensity of pain symptoms was noted to increase after the treatment, and in 1 (4%) patient in group 1 it did not change.
Discussion
Diabetes has increasingly been becoming a serious health problem on a global scale. One of the best ways to reduce mortality and morbidity among patients suffering from complications of diabetes, including diabetic foot syndrome, is early diagnosis [4, 13, 34]. Prevention and early detection of diabetes through multidisciplinary, guideline- and experience-based care is key to reducing the incidence of DFS [35–37].
In the present study, patients from the group treated topically with O3 at a higher ozone concentration of 60 µg/ml demonstrated a significantly higher rate of ulcer healing compared to the group treated with O3 applied at a lower concentration of 30 µg/ml. After the end of treatment, a significant reduction in pain was also noted in the group of patients treated with O3 at a higher concentration. No possible toxic effects of O3 were observed in any of the examined groups.
Based on the review by Fitzpatrick et al., it has been shown that O3 concentrations ranging from 5 to 60 mg/l have been determined to be safe and effective in the treatment of skin wounds, in accordance with medical standards [38]. Moreover, other authors point to the beneficial therapeutic effects of O3 at concentrations exceeding 60 mg/l, which may require more intensive studies to analyse the effects of its toxicity [27, 29]. Currently, no studies provide a clear answer as to why different O3 concentrations are more or less effective on human tissues. One plausible explanation may be that ozone does not damage the eukaryotic cell wall of the host. This is probably due to the ability of human cells to compensate better for oxidative load than for pathogenic microbial cells in the therapeutic O3 concentration range of 5–-60 mg/l.
There is little information provided in the available literature regarding the optimal dosage of O3 used in gaseous form in the local treatment of DFS. This limits the translation of the results into evidence-based clinical practice. Routine is required, along with universal reporting of O3 dosing parameters in future studies, in order to optimize the therapeutic effects of this method and to enable uniform use of precise concentrations in clinical practice.
Among the increasingly more often proposed forms of treatment for diabetes and its complications, including DFS, there are selected physical treatments, including O3 therapy [21, 37, 38]. O3 applied topically in the form of a gas mixture has a direct disinfecting and trophic effect. By stimulating the formation of peroxides, it exerts a systemic antibacterial and antiviral effect; increases the deformation of red blood cells, thus improving blood circulation; promotes the supply of oxygen to tissues; increases the efficiency of glucose metabolism, in erythrocytes among others. Finally, thanks to the activation of antioxidant enzymes responsible for the elimination of peroxides and free radicals, it improves the metabolism of fatty acids, supporting the repair mechanisms of treated tissues [20, 24, 39].
Some studies suggest that O3 therapy is more effective than standard treatment for DFS. An example may be provided by the results of the work of Kushmakov et al. who emphasize that thanks to the rapidly developing clinical research on O3 therapy and its rapid inclusion in comprehensive treatment procedures, O3 therapy has been gaining popularity and is at the forefront of DFS treatment methods [18]. In the study of Sun et al. conducted in patients with DFS, the O3 therapy group had a higher wound healing rate after a 12-week follow-up as compared to controls (p < 0.05). Kaplan-Meier curves indicated a higher cumulative wound healing rate in the O3 therapy group (p < 0.05). Additionally, the O3 therapy group had less in-patient days, lower duration of antibiotics application, re-infection rate, and readmission rate compared to the control group (p < 0.05) [37]. In another study, Lavery et al. has indicated that O3, as a topical treatment for infected skin wounds, deserves serious consideration and further research. It can be used as an alternative to traditional forms of therapy due to its broad antimicrobial activity profile, strong wound healing stimulating effect, and relatively low treatment costs [40]. The observations presented above may be confirmed by the study conducted by Astasio-Picado et al., i.e. a review of the following databases: PubMed (Medline), Dialnet, Google Scholar, Web of Science (WOS), SciELO and Scopus, which considered articles published between November 2014 and June 2023. The results of the analysed studies proved O3 therapy to be a promising method in the treatment of wounds in patients with DFS, due to its high therapeutic effectiveness and low number of side effects [41].
There are also studies indicating that O3 therapy or hyperbaric oxygen therapy have no significant effect on wound healing in DFUs [19, 42].
The Italian Scientific Society of Oxygen-Ozone Therapy (SIOOT), in Bergamo (Italy), suggests using a range of O3 concentration from 10 to 50 µg/ml (very rarely 60–80 µg/ml) in different approaches (or administration routes), with which O3 is introduced into the patient body: minor autohemotherapy, infiltration of ozonated blood, major autohemotherapy, rectal ozone, topical ozone in lipophilic media, ozonated water or oil. In the oxygen-ozone major autohemotherapy (O2-O3-MAHT), where usually the concentration of 45 µg/ml O3 (in the O2-O3 gaseous mixture) is introduced in the autologous blood (200 ml), previously withdrawn in a sterile bag and then reintroduced, the “actual” concentration of O3 entering the blood depends on the medium (plasma water) and on blood temperature (about 37°C). So, following Henry’s law, if in air there is 1% O3 = 7.824 µg/ml O3 at 25°C, then 45 µg/ml of O3 in the preparation mixture is the equivalent of 5.75% O3 (weight), yet in water and at 37°C the O3 concentration should drop to less than 8 µg/ml, actually amounting to 7.165 µg/ml [27].
In line with the current recommendations of scientific societies, and thanks to appropriately developed guidelines regarding the methodology of treatments and the quality of devices for generating O3, O3 therapy may in the future play a significant role in the treatment of chronic wounds, constituting a safe and effective alternative method of treating DFS, especially in the light of the growing resistance of pathogenic bacteria to the antibiotics used [4, 5, 14].
Therefore, in order to introduce O3 therapy into the set of routine treatment methods for DFS, it is necessary to conduct multi-centre, randomized, double-blind clinical trials on large patient populations, in order to clearly determine the optimal concentrations and duration of exposure to O3 and, consequently, develop the standardization of recommendations and clinical practice guidelines in this regard.
The strength of the study is the collection of complete data and the controlled course of the study, which allows obtaining reliable results. Although this is a pilot study, it initially confirmed the clinical usefulness of local ozone therapy treatments in the management of DFS, which is generally very resistant to previously used treatment methods. The data presented in this study indicate that ozone therapy as a form of local treatment of infected skin wounds which deserves further research as an alternative to traditional therapies due to its potentially high therapeutic effectiveness, lack of significant side effects and relatively low cost of treatment. The authors believe that supportive treatment using local ozone application, after confirming the presented preliminary results in randomized trials on larger patient populations, will in the future constitute an element of comprehensive therapeutic treatment for patients with diabetic foot syndrome.
The study has some limitations. Firstly, it is a pilot study (with a limited number of patients studied), while prospective randomized controlled studies provide stronger evidence. Secondly, the study was conducted at a single centre. Lastly, our follow-up period was limited to 10 weeks, and longer-term follow-up would be of greater significance. The study is characterized by absence of a control group of patients, which would have allowed for comparison with patients of similar characteristics treated with the use of routine methods. It seems that the value of the conducted research would also be increased by a comparison of neuropathic and ischemic foot ulcers, which was not possible in this study due to the limited sample size. The sample size was not calculated in the analyzed group of patients. The study also did not include the analysis of previous standards of care.
Conclusions
The study reported here investigated the short-term effects of local O3 therapy in the treatment of DFS, depending on the ozone dose concentration. Higher doses of O3 resulted in faster healing of ulcers and reduced pain. The possibility of using higher O3 dose concentrations is therefore worth considering because the results appear to be encouraging. Therefore, promoting the clinical use of O3 therapy in the treatment of DFS is justified.