Introduction
In recent years, it has become clear in case of the development of specific branches of oncology that not only early detection and extensive diagnostics, but also properly conducted monitoring and follow-up are important for a significant reduction in the percentage of mortality rate in the group of patients treated for various types of cancer [1, 2]. Properly conducted follow-up usually allows for earlier detection of possible recurrence or occurrence of other adverse events and complications after initial treatment conducted [3, 4]. In many cases, however, thanks to a properly conducted follow-up, it is possible to detect another parallel cancer occurrence in the same patient [5]. In case of melanoma, many publications and multicentre analyses have demonstrated the possibility of concomitant occurrence of other types of cancer during follow-up period after primary treatment [6]. There are also reports that indicate the possibility of a familial predisposition to cancers associated with occurrence of melanoma. The coincidence is mainly related to: breast, prostate, colorectal, skin and nervous system cancers, acute myeloid leukaemia/myelofibrosis and Waldenström’s macroglobulinemia/myeloma [7]. Unfortunately, despite such extensive knowledge on this subject, the proper follow-up of patients treated for melanoma is not consistent in different countries, and further research on larger patient populations is necessary to better define guidelines [8].
Aim
The aim of this study was to analyse the coincidence of skin cancers, precancerous lesions and other cancers diagnosed during the follow-up among patients with melanoma.
Material and methods
In this study, all patients treated between January 2019 and December 2022 (both surgically and conservatively) due to melanoma in the Lower Silesian Oncology, Pulmonology and Haematology Centre were included. Patients’ medical history was analysed for occurrence of other skin cancers or precancerous lesions confirmed by biopsy after melanoma surgical excision. The data were supplemented with solid neoplasms occurring in the study population before and after melanoma diagnosis. Skin lesions considered in this study were: another primary melanoma (including melanoma in situ, and invasive melanoma), basal cell carcinoma, squamous cell carcinoma (including Bowen disease), dysplastic nevus, and actinic keratosis. Factors included in comparison of the population in which skin lesions were diagnosed and in those in which they were not included: sex, age, location of primary melanoma and T stage of primary melanoma.
In our Institution, all patients diagnosed with melanoma had total body dermoscopy at the end of the primary treatment. Patients diagnosed with Tis and T1a melanoma had dermoscopy once a year in local dermatology departments. Patients with T1b-T4b melanoma remained under Cancer Centre control. Dermoscopy was not performed routinely during follow-up, however new lesions were assessed based on the patients’ findings (patients were trained to perform a self-examination at least once a month). Follow-up was done in accordance with recommendations of the Polish Society of Oncology [9]. In early melanoma (melanoma in situ, and T1 stage) the follow-up visit was once or twice a year. In locally advanced melanoma without nodal metastasis (T2 – T4 stage, N0 stage) the follow-up visit was every 3–6 months for the first 2–3 years, and thereafter every 6–12 months. Patients with nodal metastasis, local recurrence, satellitosis, in-transit metastasis or in active surveillance after confirmed nodal metastases (any T stage, N1 – N3 stage) had visit every 3–4 months for the first 2 years, and every 3–6 months for the next 3 years. In patients with distant metastases the interval between visits was determined individually. The median observation time was 18.2 months.
Results
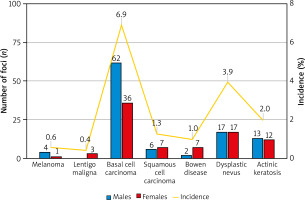
Out of 709 patients, in 14 patients, 24 co-lesions were excised during primary resection of melanoma. In 86 patients, another 160 skin lesions were surgically excised during follow-up. Median time between diagnosis of melanoma and the excision of suspected lesion was 126 days. In 61 patients there was one site of the skin lesion, and in 39 patients at least two. In majority (87.0%), there was only one type of excised lesion per patient, however, in 13 (13.0%) patients there were two or three considered types of lesions. During follow-up, in 7 patients, 8 new primary melanoma (including 3 lentigo maligna) were diagnosed (as shown in Table 1). 98 basal cell carcinoma (BCC) and 22 squamous cell carcinoma (SCC) foci were excised in 49 and 16 patients, respectively. The analysis also covered dysplastic nevi and actinic keratosis, which were diagnosed in 28 (34 foci), and 14 patients (25 foci), respectively. The proportions of skin lesions broken down by gender and incidence were presented in Figure 1.
Table 1
Occurrence of skin lesions confirmed by biopsy after diagnosed melanoma
Figure 1
Gender structure of the biopsy-confirmed foci of the skin lesions and the incident rate expressed as the percentage of people in whom lesions were detected to the whole group
Other cancers occurred 29 times in 28 patients (3.9%), of whom in 15 patients it was after diagnosis of melanoma. In total, there were: 7 colorectal cancers, 2 pancreatic cancers, 3 lung cancers, 4 breast cancers, 3 female genitourinary neoplasms, 1 sarcoma, 6 prostatic cancers, 2 renal, and 1 bladder cancer. The incidence in the group of patients diagnosed with skin lesions after diagnosis of melanoma was similar to the group without new lesions (3.0% vs. 3.4%).
The mean age was 59.9 ±16.6 years in the whole group. In patients diagnosed only with primary melanoma and in those diagnosed with other skin cancer after melanoma, the mean age was 58.7 ±16.4 and 67.3 ±15.6 years, respectively (p < 0.001). In total, 354 (48.2%) men and 379 (51.6%) women were enrolled in this analysis. Among the patients in the group with new skin lesions, there were more men (55% vs. 47.3%), however, these differences were not statistically significant (p = 0.153). In addition, a shift in the distribution of the T stage of primary melanoma towards earlier tumour advancement, especially melanoma in situ (20.0% vs 7.9%), was observed in the group with detected skin lesions (p = 0.010). Melanoma located primarily on the head or neck accounted for a higher proportion of patients with subsequent skin lesions, however, no significant difference was observed in comparison with melanoma patients without diagnosed skin lesions (p = 0.056). 22 out of 100 patients (22.0%) who had later diagnosed skin lesions, and 182 out of 609 patients (29.9%) who had no skin lesion found, underwent at least one cycle of systematic treatment. Groups did not differ significantly considering the administered chemotherapy after melanoma (p = 0.106) (Table 2).
Table 2
Comparison of patients with new skin lesions and without skin lesions
Discussion
The basis for post-treatment follow-up among melanoma patients is the assessment of scars after excision of the primary focus, recurrence or in-transit metastases, as well as the assessment of regional lymph nodes [10]. In this process, the patient should actively participate, because in more than half of the cases may be the first person noting possible pathologies during the self-examination [11]. However, as the analysis above has shown, other skin cancers to which attention must be paid may be detected during this period. The reason for including dysplastic nevi and AK foci in the analysis was twofold: firstly, these are precancerous lesions with relatively high risk of progression [12], and secondly, they have been used as indicators as they can be misdiagnosed as skin cancers [13]. In conclusion, more than half of removed lesions in the study group had significant clinical consequences for the patient, and the rest were clinically not significant.
The risk of melanoma recurrence is highest in the first 3 years from diagnosis, therefore it is postulated to increase the frequency of follow-up visits in this period [14]. Despite the multitude of recommendations regarding the follow-up of a patient after diagnosed melanoma, both the time intervals between visits and the utilization of individual imaging studies remain the subject of debate [15].
Patients with diagnosed melanoma have higher chance of developing both subsequent melanoma and other cancers [16]. Despite different signalling pathways, disturbance of which causes formation of other cancers, there is a coincidence of their occurrence due to similar risk factors, among which exposure to UV light, lifestyle and age are considered to be the most important [17, 18]. There are reports of varying strengths of evidence for correlations of skin cancer induction with drugs and in recent years rheumatological treatment has been indicated as an area requiring special attention in terms of skin cancer induction [19]. What is more important BRAF inhibitors, commonly used in advanced or metastatic melanoma treatment, may also induce precancerous lesions and SCC [18, 20].
The analysis covered a relatively large group for a single-centre study consisting of patients treated at different stages of the disease, being a cross-section of patients suffering from melanoma in Eastern Europe.
Conclusions
Melanoma patients during surveillance should be under close supervision not only due to the potential recurrence, but also due to the high risk of novel or subsequent skin cancer lesions. In this study it was shown that BCC and SCC are the most commonly diagnosed lesions among melanoma patients during follow-up. That aspect should be taken into account when creating recommendations for melanoma follow-up. In addition, this knowledge may be useful in further research aimed at understanding the phenomenon of coincidence of these types of cancers and will better protect patients treated for melanoma in the future.








