Introduction
Recently a growing interest concerning the possible relationship between acne and insulin resistance (IR) has been noticed. It seems that acne being a chronic inflammatory skin disease might be strongly associated with IR [1]. When diagnosing this disease as well as composing the treatment strategy, IR evaluation seems to be highly important. Both disorders share the same signal transduction pathways, i.e. mammalian target of rapamycin kinase 1 (mTORC1) and insulin-like growth factor-1 (IGF-1) [2]. Nevertheless, correlating acne with IR still remains a diagnostic challenge. Various methodologies for IR evaluation have been proposed, such as euglycemic metabolic clamp, Homeostasis Model Assessment (HOMA-index) and Quantitative Insulin Sensitivity Check Index (QUICKI) [3]. Clinical severity of acne may be evaluated with scoring systems such as GAGS (Global Acne Grading System) [4] or AFAST (Adult Female Acne Scoring Tool) [5]. Still, there is no method, which would provide a certain diagnosis of both coexisting IR and acne in a single test.
According to the updated literature, acne affects more than 85% of teenagers [2], and even highly sophisticated therapeutic modalities are sometimes not effective. In addition, lots of attention have been paid to various side-effects of the acne therapy and many research projects have been focused on the topic of reduction in adverse events, while new targeted acne treatment remained unsolved [6]. Due to certain pathogenetic similarities between acne and IR, proper treatment of IR might result in clinical improvement of acne.
The aim of our review was to evaluate a possible role of IR in the etiopathogenesis of acne as well as to analyse the efficacy of IR treatment being one of the therapeutic modalities within the treatment strategy of acne patients.
Acne
Acne is a relatively common chronic inflammatory skin disease involving folliculopilosebaceous unit. Women and adolescents constitute the majority of patients affected by acne [1]. Skin lesions such as comedones, papules, pustules or nodules are generally located within the skin of the face, shoulders, back and chest [1]. Widely-known acne is divided into three categories, related to the severity of the disease: mild, moderate and severe [4].
Acne is a very heterogeneous disorder associated with highly complex etiopathology. It includes excess production of sebum (hyperseborrhea), hyperkeratosis and inflammation [7]. Many transcription factors such as forkhead box protein O1 (FoxO1), 1,25-dihydroxyvitamin D and calcium are connected with sebum production [8].
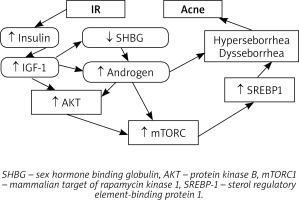
Abnormal hormones function – hyperandrogenemia, hyperinsulinemia and an elevated level of insulin growth factor-1 (IGF-1) have the crucial role in the acne development as well. Hyperinsulinemia is responsible for increased proliferation and dysfunction of keratinocytes by stimulation of IGF-1 receptors. Hypersecretion of IGF-1 leads to abnormal sebum production, hyperproliferation of sebocytes and lipogenesis [1]. The synthesis of androgens is promoted by insulin and IGF-1. Moreover, IGF-1 enhances androgen receptor signal transduction. As a consequence, the elevated androgen level encourages hyperseborrhea [1].
Acne vulgaris tends to be regarded as a disease of Western civilization due to the fact that the increased serum level of insulin/IGF-1 is in addition significantly correlated with a high intake of carbohydrates, insulinotropic milk and dairy products [2]. In the study published by Cerman et al., higher dietary glycaemic load and milk consumption in acne patients in comparison to the control group have been proven [9], whereas acne has not been observed in populations with a Palaeolithic type of eating habits [2]. The hyperglycaemic diet activates mTORC1 leading to both dysfunction of the sebaceous gland and development of IR via ribosomal protein S6 kinase β1 (S6K1) [1] and supresses FoxO1, which is responsible for repressing the androgen receptor, androgen signalling, synthesis of hepatic IGF-1 and regulating lipogenesis. Inactivated, does not influence on mTORC1 [1]. Consumption of a lower amount of products mentioned above decreases severity of inflammation, lowers production of proinflammatory chemokines and reduces the size of sebaceous glands [1]. The relationship between acne and lifestyle habits has been further investigated by many researchers. Stewart et al. have recorded statistically significant concomitance of vitamin D deficit and a positive tissue transglutaminase antibody serum level whereas no correlation of the vegan diet or increased body mass index (BMI) with acne development has been proven [10]. Snast et al. confirmed that overweight and obesity are inversely associated with acne [11]. Moreover, a positive correlation between the higher BMI and severity of acne has been recorded [12].
The role of Cutibacterium acnes in the pathogenesis of acne still remains unclear. There is no significant difference in terms of skin colonization with C. acnes between acne patients and patients without signs and symptoms of the disease. Therefore, it has been absolutely clear already for a long time that C. acnes and proliferation of this microbe within the skin of patients are not the basic causative factors in acne [8, 13]. Nevertheless, abnormal load of C. acnes is linked to sebaceous follicles due to a high concentration of lipids in this area [1]. S. epidermidis inhibits proliferation of C. acnes therefore modification of skin microbiota is considered to be a logical treatment modality in acne [13].
Acne may also occur in the course of many endocrinological disorders. An intensified sebum excretion rate in patients with acromegaly was observed by Burton et al. in 1972 [14]. Growth hormone (GH) induces production of IGF-1 stimulating lipogenesis and 5α-reductase. Testosterone and DHT formed in excess lead to enlargement of the sebaceous gland, excessive sebum secretion and therefore provoking acne skin lesions [15]. The reverse situation is presented by patients suffering from Laron syndrome characterized by IGF-deficiency. It has been observed that a significant lack of IGF prevented development of acne [16].
It has been recorded that structural similarity between GH and prolactin may cause IR in the course of hyperprolactinemia [17]. Langan et al. have indicated that prolactin itself may stimulate sebocytes proliferation [18].
Most of the patients with congenital adrenal dysplasia (CAH) present a specific defect of 21-hydroxylase which prompts the deficit of cortisol and aldosterone [19]. It leads to ACTH production increase and stimulation of excessive adrenal androgens production. Treatment of CAH is based on supplementation of glucocorticoids which can stimulate expression of Toll-like receptors, which bind the peptidoglycan structure of the C. acnes cell wall, and finally triggering the immune response [15].
The most common disorders associated with polycystic ovary syndrome (PCOS) are hyperandrogenism, dysfunction of the menstrual cycle and polycystic ovarian morphology [20].
However, acne in PCOS patients may be triggered not only by excessive androgen production, but also by presence of IR which often coexists with PCOS [20].
Insulin resistance
IR is defined as an inability of insulin to provide appropriate glucose transport into metabolic tissues such as the skeletal muscle, adipose tissue, and liver, despite its normal or elevated secretion. As a consequence, IR is responsible for development of hyperglycaemia and hyperinsulinemia [17]. IR may be caused both by genetic and environmental factors [21]. Up till now various genetic loci related to the increased risk of IR have been identified. So have been those associated with glucose metabolism, insulin activities, signalling in insulin-dependent tissues or insulin and IGF receptors activation [22].
A wide spectrum of various etiopathological factors in the development of IR have been reported. Obesity seems to be one of the most common [23, 24]. In addition, chronic inflammation [25, 26], hypertriglyceridemia, low HDL serum concentration [27], mitochondrial dysfunction [28], gut microbiota [29], the excess activity of antagonistic hormones such as cortisol, glucagon, thyroid hormones seems to play a significant role [17]. The high risk of developing IR and hyperinsulinemia is associated with certain living habits such as smoking [30], and a high intake of milk and carbohydrates [2].
IR is included as a component of the metabolic syndrome [31] and it is an independent risk factor for cardiovascular diseases [28, 32], type 2 diabetes [26, 32] and non-alcoholic fatty liver disease [33]. It may coexist also with impaired glucose tolerance or hypertension [17]. IR is also associated with the whole variety of endocrine disorders such as acromegaly, hyperprolactinemia, hypercortisolism, hypopituitarism, hyper- and hypothyroidism, primary hyperparathyroidism, pheochromocytoma, primary aldosteronism, congenital adrenal hyperplasia (CAH), PCOS and hypogonadism [17].
Diagnostics of IR is rather complicated. Euglycemic metabolic clamp described by R. A. DeFronzo, J. D. Tobin and R. Andres in 1979 is regarded to be the golden standard. It requires constant intravenous infusions of insulin to suppress the function of B-cells, in parallel to intravenous glucose infusion administered to achieve the status of euglycaemia. The amount of infusions corresponds with glucose uptake by peripheral tissues. Insulin resistance is calculated based on the number of glucose infusates provided and body weight. Euglycemic metabolic clamp enables assessment of peripheral tissue as well as B-cell sensitivity to insulin [34, 35]. Unfortunately the clinical value of this methodology is limited due to its complexity.
Homeostasis Model Assessment (HOMA) is generally one of the basic and frequently used methods in terms of IR diagnostics. This method is based on evaluation of serum levels of both fasting glucose and insulin. D. R. Matthews et al. have proposed to collect three blood samples in 5 min’ intervals in order to improve the quality of results obtained [34, 35]. Further an “insulin × glucose/22.5” formula of calculation is recommended. Unfortunately a standardised cut-off value is still not established and therefore we are facing significant difficulties using HOMA methodology in our clinical practice. Geloneze et al. have identified the cut-off values above 2.7 HOMA1-IR and above 1.8 HOMA2-IR [36]. For the study on the Polish population, Szurkowska et al. have considered IR cut-off values as above 2.1 HOMA-IR [37]. Gayoso-Diz et al. in their study have suggested that the cut-off value should be established in relation to the age and gender of investigated patients [38].
Another widely-used method is the Quantitative Insulin Sensitivity Check Index (QUICKI). It considers fasting insulin and glucose serum level measurements as well and a significant correlation with glucose clamp has been proven [3]. Unfortunately different cut-off values are being set by different laboratories due to various methodology applied for insulin serum level measurements [39]. Matsuda index considers not only fasting glucose and insulin serum level measurements, but also their mean concentrations during the oral glucose tolerance test (OGTT) measured 120 min and 180 min from the 75 g glucose consumption. In this case both hepatic and peripheral tissues’ sensitivity to insulin is being evaluated [39].
McAuley et al. have proposed a different type of measurements to diagnose IR in the general population. According to the authors, serum levels of fasting insulin and triglycerides provide the best predictive value [40].
A wide range of methods available to select from may also be responsible for certain difficulties in the process of IR diagnostics. HOMA-IR, despite its wide application in scientific research and significant correlation with the glucose clamp, presents a relatively limited importance in the clinical practice due to unclear standardized cut-off values. There are no typical clinical symptoms of IR. Regardless of a strong correlation between metabolic syndrome and IR, patients with normal BMI may also suffer from IR [41].
Relationship between acne and insulin resistance
As mentioned above, acne seems to be strongly associated with IR [1] but the role of IR in the pathogenesis of acne is still not clearly understood. High glycaemia induces both synthesis and secretion of insulin, which encourages ovaries and adrenal glands to produce androgens. It can also lower the serum level of sex hormone-binding globulin (SHBG) and therefore strongly intensify androgen activity and facilitate development of acne [42]. Insulin also decreases IGF-1 binding protein, which causes a significant rise of the free IGF-1 serum level [15]. Smith et al. have demonstrated that IGF-1 stimulates lipogenesis in SEB-1 sebocytes through activation of the Phosphoinositide 3-kinase (PI3-K)/Akt pathway, which leads to the enhanced expression of sterol regulatory element-binding protein-1 (SREBP-1) [43].
In patients with acne, an increased mTORC1 activity has been detected, which is strongly associated with IR, obesity, type 2 diabetes and cancers such as melanoma and prostate cancer [2]. IR seems to be induced via insulin receptor substrate-1 phosphorylation due to the activation of S6K1 kinase related to an increased activity of mTORC1. Also, a significant correlation between a decreased expression of insulin, IGF-1 and mTORC1 and a reduced prevalence rate of acne has been observed [2].
In case of young males, IR in the course of acne has been reported in the literature. Nagpal et al. have noticed a significant correlation between HOMA-IR value and acne development. However, no statistically significant difference between HOMA-IR value, metabolic syndrome and acne severity has been observed [44].
In the study of Del Prete et al. performed in the male population of acne patients, a significant correlation between the acne score and HOMA-IR has been noticed, with exclusion of any possible impact of the abnormal androgenic profile, evaluated based on measurements of serum levels of free testosterone, total testosterone, dehydroepiandrosterone sulfate (DHEAS) and SHBG. Therefore, it has been concluded that acne might have been caused only by hyperinsulinemia, and not by intensified androgen activity [45]. Kartal et al. have reported that IR is a risk factor for acne which is independent of hyperandrogenemia as well [46]. Likewise Emiroglu et al. have received the results of a significantly higher HOMA value in male and female acne patients (HOMA = 2.87), and the value suggested that the IR had already developed [16]. Whereas Cerman et al. have found lower adiponectin levels in acne patients [9]. Adiponectin increases insulin sensitivity, and so its deficit is a risk factor for IR development (Table 1).
Table 1
A significant correlation between HOMA values and the incidence of acne has been reported by Emiroglu et al., Nagpal et al. and Del Prete et al.
However, some studies have reported no correlation between IR and acne development. Balta et al. have found no significant differences in the serum concentrations of fasting blood glucose, fasting insulin and HOMA-IR value between patients suffering from post-adolescent acne and the control group [47]. Cetinözman et al. have not concluded any association between severe acne and hyperandrogenemia or insulin resistance as well [48] (Figure 1).
Treatment of acne
Treatment methods depend on the clinical features and variants of acne [49]. Severity of acne and previous treatment have an influence on modality of the therapy as well. Some patients may be satisfied with topical treatment only, however, systemic treatment or both methods combined usually bring more satisfactory effects.
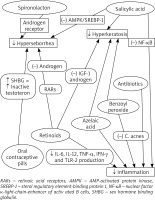
In the treatment of comedonal acne, topical retinoid is recommended. Adapalene is preferred over tretinoin or isotretinoin [49]. Azelaic acid (AA) and benzoyl peroxide (BPO) can be considered as well [49] due to their comedolytic and antibacterial properties [50–52]. Moreover, AA can be used during pregnancy and breastfeeding [53], whereas adapalene and BPO may prompt photosensitivity and skin irritations, therefore should be used with caution [54].
Mild to moderate papulopustular acne can also be treated with topical medications. The combinations of adapalene + BPO or BPO + clindamycin are strongly recommended. The monotherapy of AA, BPO or topical retinoid may be prescribed as well. Topical antibiotic + tretinoin or systemic antibiotic + adapalene therapies can be induced, however, each antibiotic prescription should be well considered due to the potential risk of antibiotic resistance development in local C. acnes and other cutaneous bacteria including staphylococci [49]. Resistance to clindamycin and erythromycin is more common than resistance to tetracyclines [49]. Despite the side effect of photosensitivity, doxycycline and lymecycline are recommended, however their use is limited to the period of 3 months [49].
Severe papulopustular, nodular and conglobate acne requires oral isotretinoin as the first-choice treatment [49]. Retinoids lower sebum production by affecting retinoids acid receptors (RARs) [55]. They also have comedolytic properties and suppress formation of new lesions [51]. By stimulating the expression of p53, retinoids lead to an increased serum level of IGF binding protein 3 and reduced androgen and IGF-1 receptors formation [56]. Retinoids display anti-inflammatory activity by inhibiting secretion of cytokines such as IL-6, IL-12, TNF-α, IFN-γ and TLR-2 production in monocytes [57]. Furthermore they suppress the expression of transcription factor activator protein (AP-1) and vascular cell adhesion molecule-1 gene [57]. Incontrovertible disadvantages of retinoids are side effects. Oral isotretinoin treatment requires periodic assessment of the liver function (liver enzymes, lipidogram) and birth control due to teratogenicity. A study performed by Dikicier has shown that most of the patients give up retinoid treatment due to its side effects [58]. Some researchers have pointed that a small dose of retinoids may boost sebocytes [55]. Soyuduru et al. have suggested that 5 months’ isotretinoin therapy leads to insulin resistance, and it is not related with age, BMI or lipid levels [59].
Systemic antibiotics with BPO, AA or adapalene are also recommended in the severe acne treatment. Hormonal antiandrogens combined with systemic antibiotics and topical preparations constitute an alternative for women. Oral contraceptive pills (OCPs) increase the level of SHBG which connects with testosterone leading to its inactivation [51]. We cannot unanimously state which progestin type involving chlormadinone acetate, cyproterone acetate, levonorgestrel or desogestrel brings the most satisfactory acne improvement [49].
AA, topical retinoid, adapalene + BPO, low-dose systemic isotretinoin or hormonal treatment may be considered as the maintenance therapy as well [49] (Figure 2).
Metformin
Metformin has a positive impact on glucose transporter type 4 (GLUT4) by increasing the expression of GLUT4 mRNA [60] and suppressing endocytosis of GLUT4 via AMP-activated protein kinase (AMPK) pathway [61]. Both mechanisms lead to a rise in GLUT4 and therefore insulin sensitivity enhancement, reducing the serum insulin level and its effects. Activation of AMPK inhibits mTORC1 which is upregulated in acneic skin and due to that suppresses sebum overproduction, which is beneficial to acne treatment [57]. Metformin regulates glycaemia not only by affecting liver metabolism, but also by increasing glucose utilization in muscles and adipocytes when elevating the level of glucagon-like peptide-1 (GLP-1) [62]. It can also suppress proinflammatory cytokine secretion and inhibit differentiation of monocytes, which lead to limitation of the inflammation [62]. Metformin reduces high serum levels of IGF-1 and androgen in women with PCOS [63]. As we have mentioned before, IGF-1 stimulates hypersecretion of sebum and therefore promotes development of acne. The obvious contribution of high-glycaemic load diet, milk consumption and lack of physical activity to the development of IR must be considered in the treatment strategy. Modification of lifestyle factors is a significant component of the IR therapy [2, 17, 21].
Table 2 presents data regarding to the effectiveness of metformin treatment modality to the patients suffering from acne vulgaris [64–67].
Table 2
Review of the effectiveness of metformin treatment in acne vulgaris patients [64–67]
All the data presented have demonstrated the effectiveness of metformin as an adjuvant therapy of acne. Only slight gastrointestinal side effects of this type of therapy have been observed.
It seems that only oral treatment with isotretinoin might cause a more significant reduction in GAGS value, however this phenomenon does not apply to PCOS patients. Moreover, metformin, contrary to oral isotretinoin, presents a positive metabolic characteristics.
Metformin does play a role not only in cutaneous disorders linked with hyperinsulinemia and hyperandrogenism such as acne or hirsutism, but also the treatment of acanthosis nigricans, eruptive xanthomas, hidradenitis suppurativa, psoriasis or skin cancers is proposed [68]. For hyperpigmentary lesions topical preparations of metformin could be applied [68]. Topical formulation throughout local suppression of the mTORC1 overexpression seems to be effective in the treatment of acne lesions in the future as well [69].
Conclusions
In our review paper a specific and possible relationship between acne development and IR has been presented on the basis of updated literature data. Increased mTORC1 signalling observed in both conditions mentioned above, seems to be an important factor and perhaps should be considered in the complex structure of treatment strategy in patients suffering from acne. Also, IR might be a possible causative factor of resistance to a standard acne therapy in our clinical practice. Moreover, oral treatment with isotretinoin may induce IR, which might be responsible for relapses of acne after discontinuation of systemic isotretinoin therapy. Acne should be obviously considered to be a systemic disease, which requires a complex and personalised therapy. Metformin seems to be an effective and interesting therapeutic modality for patients with acne. Treatment with metformin not only reduces GAGS value, stabilisation of the lipid profile, as well as serum glucose and insulin levels have also been recorded. Therefore it seems to be important to consider metformin to be not only an adjuvant medication in the treatment of acne, but also an element of the preventive approach in terms of relapses of the disease. Obviously further studies including a bigger population of patients are required in order to prove this therapeutic modality to be an effective and safe element or alternative therapy in the daily, dermatological, clinical practice.