Linear IgA bullous dermatosis (LABD) is an autoimmune subepidermal blistering disease characterized by linear deposits of IgA along the basement membrane zone. Some drugs, including vaccines, have been reported to be potential inducers of this condition [1]. Here, we describe a child with LABD following coronavirus disease 2019 (COVID-19) vaccination.
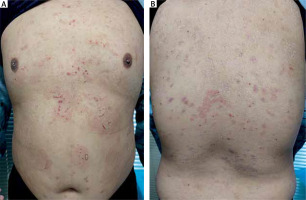
A 12-year-old boy presented with a 2-month history of a bullous eruption, which first appeared 3 weeks after a second dose of the inactivated WIBP-CorV vaccine. Prior to vaccination, the patient was healthy and without any history of skin disease or recent medication. Physical examination revealed dense erythema throughout the trunk, back and thighs as well as annular erythematous lesions with a ring of vesicles on the abdomen, which is frequently referred to as a “crown of jewels” or “string of pearls” (Figure 1). No erosion was found in the oral mucosa. Histopathological examination of the skin lesions showed subepidermal blistering with neutrophil infiltration in the papillary dermis and lymphocyte and neutrophil infiltration around the vessels of the superficial dermis. Direct immunofluorescence revealed IgA and complement 3 deposited in a linear pattern at the dermoepidermal junction (Figure 2). Indirect immunofluorescence on normal human skin was negative for IgG and IgA. The results of IgG ELISA for BP180, BP230 and type VII collagen were all negative. Immunoblotting analyses and indirect immunofluorescence on salt-split skin for IgA antibody were not performed because the patient’s serum was insufficient to complete the tests. With these findings, we confirmed the clinical diagnosis of LABD, which was treated with prednisolone (16 mg/day) and dapsone (100 mg/day). The skin lesions completely resolved within 2 weeks. Subsequently, prednisolone was stopped after another week and the patient treated with dapsone alone. One month later, dapsone was stopped. No recurrence was detected after 2 years of follow-up.
Figure 2
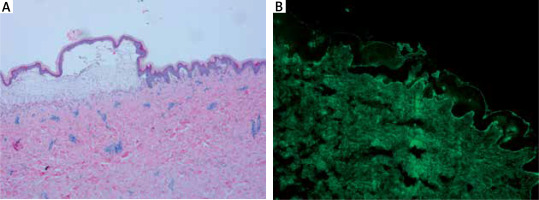
Histopathological examination of the skin lesions showed subepidermal blistering with neutrophil infiltration in the papillary dermis and lymphocyte and neutrophil infiltration around the vessels of the superficial dermis (A). Direct immunofluorescence showing linear IgA deposition at the dermoepidermal junction (B)
Autoimmune bullous diseases (AIBDs) after COVID-19 vaccines have been reported, including bullous pemphigoid (BP), pemphigus vulgaris (PV) and LABD [1]. In all cases, the most common type of AIBD was BP, followed by PV. Wu et al. [2] recently reviewed the new onset and exacerbation of AIBD following COVID-19 vaccination. The new-onset group comprised 229 patients, including 174 BP, 42 pemphigus, 4 LABD, 3 mucous membrane pemphigoid, 1 pemphigoid gestation, and 5 unidentified cases. In this group, the most common vaccine that induced AIBD was the BioNTech/Pfizer vaccine, followed by the Moderna and Oxford-AstraZeneca vaccines. The flare group included 4 LABD patients [3–7] aged 61–86 years, of whom 3 were men and 1 was a woman. The clinical manifestation of the LABD patients appeared after 1–5 days of the second or third dose of COVID-19 vaccine. After 20 days to 3 months of treatment, all patients improved or the condition was resolved. LABD can be triggered by numerous vaccinations or drugs other than against COVID-19 [8]. In our case, the clinical features appeared not to be significantly different from those previously reported. Regarding the possible explanations for AIBDs developing after vaccination, this was unclear. Coto-Segura et al. hypothesized that cross-reactions between SARS-CoV-2 spike protein antibody and tissue proteins may play a role in AIBD development [5].
The theory of molecular mimicry between specific basement membrane and epidermis proteins and the spike protein of COVID-19 vaccine was proposed as a potential mechanism. Some vaccines are suggested to activate pro-inflammatory pathways by interacting with toll-like receptors, increasing the level of cytokines, leading to the promotion of an imbalance between Th2 responses against cutaneous antigens, fostering the generation of autoreactive B cells and contributing to AIBD development [2]. However, other studies did not observe the cross-reactions between SARS-CoV-2 spike protein antibody and tissue proteins [9–11]. In addition, bystander activation appears to be involved in AIBD onset following vaccinations, particularly in patients with an associated immune predisposition [12]. Recent studies have shown that booster vaccine doses rapidly elicit a SARS-COV-2-specific immune response [13], which may have been confirmed in our case. Existing data show that a relationship between COVID-19 vaccines and AIBDs is uncertain due to the small size of samples.
To our knowledge, this is the first case of LABD in a child after inactivated COVID-19 vaccine use. In extending the application of COVID-19 vaccines to children, an increased awareness by clinicians of the cutaneous reaction to COVID-19 vaccines is crucial.