Introduction
Chlamydia trachomatis is the most common bacterial sexually transmitted pathogen with 127 million new infections among adults every year, according to WHO estimated data in 2016 [1]. However, most of the cases are infections caused by C. trachomatis serovars/genovars D-K. Lymphogranuloma venereum (LGV) is caused by C. trachomatis L1-L3 serovars/genovars and is less common [2].
Classical symptoms of LGV were originally described by Wallace in 1833 as climatic bubo, and again with more details in 1913 by Durand, Nicolas, and Favre. LGV was regarded as an endemic tropical disease and the rare cases diagnosed in Europe were considered to be imported [3]. The first epidemic outbreak in Europe was described in 2003 in men who have sex with men (MSM) in Rotterdam, when LGV emerged as a cause of severe proctitis/proctocolitis [4]. Since then, similar outbreaks, particularly among MSM, have been described in many other European countries as well as North America and Australia [2].
The L1-3 serovars/genovars are more invasive and virulent than the other C. trachomatis serovars/genovars [2]. Only one LGV case has previously been reported in Poland (in 2018) [5]. We present two new LGV cases in Poland as well as a systematic review of LGV.
Epidemiology
Conventional LGV remains endemic in some tropical and subtropical regions of South and West Africa, Southeast Asia, India, and the Caribbean. There are no global data concerning the incidence and prevalence of LGV. However, during the last 1–2 decades, an increase in the number of LGV-associated proctitis/proctocolitis cases in developed countries has been observed. According to the European Centre for Disease Prevention and Control (ECDC), almost 2000 new cases of LGV were reported in the European Union/European Economic Area (EU/EEA) in 2017 and over 2300 in 2016. A decrease in the number of cases was published for the first time since 2009. Almost all of the infections were observed in MSM, and 64% of those with known HIV status, were positive. In contrast to C. trachomatis D-K serovars/genovars infection that mainly occurs in teenagers and very young adults, 94% of the LGV patients were over 24 years of age. Moreover, 86% of cases were reported in four countries: France, the Netherlands, Spain, and the United Kingdom (UK) [6]. Most likely, the number of unrecognized and unreported cases of LGV in Europe and other parts of the world is exceedingly high, due to the lack of appropriate diagnostics and epidemiological reporting and surveillance. In Europe, North America and Australia, LGV is mainly described in MSM, particularly those who are infected with HIV. LGV has frequently been strongly associated with HIV infection. In different populations up to 100% of MSM with LGV were HIV positive, but in recent years the percentage of HIV-negative LGV patients has increased, particularly in some settings [7, 8] and associations with other STIs and hepatitis C have become stronger. HIV-negative MSM with LGV are often on Pre-Exposure Prophylaxis (PrEP). There are also limited cases of LGV described in heterosexual women, including very rare cases of proctitis [9], however, there is no evidence of LGV transmission among heterosexuals in most settings [10]. A recent study, published in 2019, revealed that almost 26% of 500 tested C. trachomatis positive rectal swabs in the UK, Austria, Croatia and Slovenia were positive for LGV [11]. In contrast, a similar study, published in 2011 from the USA, reported less than 1% positive from their samples [12].
It has not been fully elucidated why rectal infection in MSM is 15 times more common than genital infection. There have been suggestions of recto-rectal transmission. In some settings, fisting, use of sexual toys, and enema or rectal douching have increased the risk of anorectal LGV [13, 14]. There has been a hypothesis that C. trachomatis has a higher affinity for rectal than for urethral epithelium, but this has not been confirmed [15]. Another explanation of the disproportion between genital and rectal infections is that rectal mucosa has a larger surface and is more susceptible to injury.
Other risk factors of LGV include high-risk sexual behaviours: unprotected receptive anal intercourse, anonymous sexual contacts, and concurrent use of drugs like gamma-hydroxybutyrate and methamphetamines (chemsex) [13, 14].
Unprotected sex and group-sex parties can also increase the chance of a multiple spread of LGV. LGV can also reoccur, especially in patients with co-existing HIV infection and HCV coinfection [16].
Aetiology and pathogenesis
The classification of C. trachomatis types was earlier based on detection of different bacterial major outer membrane protein (MOMP) antigens (serological typing) but is now based on determination of single nucleotide polymorphism in the gene encoding for MOMP-ompA [2, 8]. The C. trachomatis serovars/genovars causing LGV are L1-L3. The most common type of C. trachomatis currently causing LGV in Europe is the subvariant L2b. L2 is in the second place in most countries; however, in France, an increase in the number of L2 and decrease in L2b infections was reported in 2013 [17]. Mixed infections with D-K and L types are observed, which probably have resulted from DNA recombination. Recently, 25 LGV cases due to a new hybrid L2b/D-Da genovar were described in Portugal. All of these isolates were collected from MSM with proctitis [18]. In another study, including MLST of C. trachomatis strains from different parts of the world, all LGV isolates belonged to ST1. Four of the LGV isolates in Western Europe were collected from women [19].
All C. trachomatis reproduce intracellularly as non-infectious, metabolically active reticular bodies that transform into smaller, less metabolically active and infectious elementary bodies that leave the cells and can survive extracellularly. At the beginning of the infection MOMP of elementary bodies attach to heparan sulfate on the host cell surface [20]. In contrast to other serovars/genovars, C. trachomatis L1-3 can replicate not only in epithelial cells but also in macrophages. This difference can be correlated with polymorphisms in the chlamydial inclusion membrane (Inc) proteins. The bacteria, together with macrophages, get into lymphatic vessels and lymph nodes, causing inflammation of lymphatic tissue [21].
Symptoms
The incubation period lasts 3 to 30 days. The primary stage of LGV begins as a small, painless papule or pustule that may evolve into a small, herpetiform ulcer [2, 4]. The lesion is localized at the site of bacterial inoculation and usually involves vulva or posterior wall of vagina or cervix in women and penis in men but can also appear in the perianal region, or in the mouth [22] or in the throat. Sometimes mucopurulent discharge from the rectum, urethra or cervix can appear. The primary symptom disappears spontaneously within 1 week and often remains unnoticed [3, 8].
The symptoms of the second stage appear about 2–6 weeks after the onset of the primary lesion and are connected with local lymphadenopathy and haematogenous dissemination of the infection. The patients can have general symptoms like fever, myalgia and arthralgia and/or gastrointestinal symptoms [23].
Rare symptoms include hepatosplenomegaly and meningoencephalitis. Reactive arthritis (SARA) has been reported in a few LGV patients, rarely including women [24].
The most common presentation of the conventional secondary stage of LGV in low and middle-income tropical countries is an acute inguinal syndrome. The patients have inguinal and/or femoral painful lymphadenopathy with inflammatory lesions on the skin overlying the affected nodules. The lymphadenopathy is usually unilateral, and the lymphatic nodes tend to form a firm, immovable mass, or abscesses (so-called bubo) sometimes with drainage. In about 10–20% of patients, the lymphadenopathy appears on both sides of the inguinal ligament (so called groove sign) that is a pathognomonic symptom of LGV. The symptoms are much more common in men [2, 23].
In most of the infected women, the vagina and cervix are involved with the drainage of the regions to the deep pelvic or retroperitoneal lymph nodes. The inflammation of the nodes is not visible or palpable, and the only symptom can be a pain in the lower back or lower abdomen. The infection can spread by lymphatic vessels from lower vagina to perirectal nodes and the rectum, which cause acute anorectal syndrome, the most common presentation of the disease in women.
Anorectal localization of the symptoms is usually observed also in MSM practicing anogenital sex. Almost all the cases of LGV reported in Europe and North America belong to this group. The patients with LGV proctitis/proctocolitis report anorectal pain and itching, bleeding and/or purulent discharge, tenesmus, constipation or diarrhoea, and pain in the lower abdomen. Anoscopy can reveal inflammation, haemorrhagic mucosal lesions, ulcers, and sometimes tumorous masses. The clinical symptoms as well as results of histopathological examination often mimic inflammatory bowel disease, especially Crohn’s disease [3, 23, 25]. Some cases have also been misdiagnosed as cancer [26].
Oral and pharyngeal infection is rare and can be connected with enlargement of cervical lymphatic nodes (that can mimic lymphoma) or tonsillitis [27]. In sporadic cases autoinoculation can lead to conjunctivitis.
The tertiary stage or so called anogenitorectal syndrome appears in untreated individuals, is more often in women and MSM, and is connected with permanent damage of affected tissues and organs with abscesses, fistulas, and scaring. Late complication in patients with proctitis is the stricture of the rectum, which can cause pain during passing stools, constipation, and, in extreme cases, megacolon. Chronic perirectal lymphangitis can lead to haemorrhoid-like swellings “lymphorrhoids.” In women recto-vaginal fistulas can appear, and chronic genital ulcers (esthiomene) are described. Another late complication of LGV can be a frozen pelvis syndrome. The organs in the pelvic cavity cannot move freely because of adhesions that are related to chronic pain. Damage and obstruction of lymphatic vessels may lead to chronic irreversible lymphedema and elephantiasis of genitalia [8, 23].
Making the diagnosis
Laboratory diagnostics of LGV is recommended in all persons with clinical symptoms suggesting LGV and in sexual partners of patients with LGV. LGV tests should also be performed in all MSM with positive C. trachomatis tests in anorectal samples, and priority should be given to HIV positive patients. C. trachomatis L serovars/genovars can be detected in swabs from genital, oral, or perianal ulcers connected with the primary stage of LGV, anorectal swabs in case of LGV proctitis and urethral swab or first void urine in case of urethritis. In patients with the second stage of the disease, enlarged lymphatic nodules or buboes aspirate can be used.
Nucleic acid amplification tests (NAATs) routinely used (or other more rarely used diagnostic assays such as direct immunofluorescence) in laboratory diagnosis of C. trachomatis, do not differentiate between D-K and L1-L3 serovars/genovars. Accordingly, the European LGV management guideline recommends commercially available NAAT detecting C. trachomatis as the first step of the LGV diagnostics. In the case of a positive test result, a NAAT detecting L1-3-specific DNA of C. trachomatis should be performed. The problem is the low accessibility of these NAATs, particularly when few commercially-available NAATs exist. Laboratory-developed “in house” NAATs are frequently used but appropriate validation and internal and external controls are needed because these NAATs can substantially differ in specificity and sensitivity [2, 8, 23]. Recently two commercial NAATs (Allplex™ Genital ulcer assay and VIASURE assay) were evaluated for detecting LGV in rectal samples. A PCR-based restriction fragment length polymorphism analysis (PCR-RFLP) of the ompA gene was used as a reference method. For both of the evaluated NAATs, about 97% of overall results, and 100% of negative results were consistent with the reference test [28].
In the absence of NAATs, chlamydia genus-specific serologic tests can be performed, but they are less sensitive and less specific than molecular diagnostics. High titres of particularly IgM antibodies in serologic tests strongly suggest the diagnosis in symptomatic patients but cannot confirm it in asymptomatic individuals and, on the other hand, low titres cannot completely exclude LGV [2, 8].
Treatment, partner management, and follow up
The first-line treatment recommended by the European and US CDC LGV management guidelines consists of doxycycline 100 mg twice daily for 21 days [2, 29]. Treatment efficacy is 98.5% (96.3–100%; I 2 = 0%) according to the largest available meta-analysis [30]. A high cure rate after this treatment is also confirmed by the European LGV management guideline [2]. The effectiveness of a shorter course of doxycycline (7–14 days) remains to be appropriately proven.
An alternative therapeutic antibiotic regimen – erythromycin 400 mg four times daily for 21 days or azithromycin 1 g once a week for 3 weeks orally (in case of azithromycin, clinical data are very limited) [2, 29]. Escape options – minocycline 300 mg loading dose, followed by 200 mg twice daily for 21 days, rifampicin 600 mg once daily for 3 weeks, and moxifloxacin 400 mg once daily for 21 days [2, 3].
Additional treatment of conventional LGV includes drainage of buboes and reconstructive surgery if needed.
HIV-positive patients do not need any different treatment for LGV. Up to present date, there is only one verified LGV case of doxycycline treatment failure, which was successfully treated with moxifloxacin [31]. There is evidence that LGV but also non-LGV rectal chlamydial infections are more challenging to cure. This especially concerns azithromycin due to a lower concentration of the antibiotic in the gastrointestinal tract than in the genital tract [32].
No test of cure (TOC) is needed if the first-line 3-week doxycycline regimen has been complied with. Only if using an alternative treatment regimen, TOC needs to be performed.
Patients should be seen at the end of the treatment for STD screening and to ensure the resolution of the lesions [2].
All patients diagnosed with LGV should also be tested for syphilis, HIV, HBV, and HCV. In case of positive tests, the patients should receive appropriate therapy [33, 34]. The sexual partners of LGV patients up to last 90 days should be notified and treated immediately with prophylaxis dosage. Prophylactic treatment is the same as recommended in infected individuals [2, 29].
Prevention
Currently, there is no successful vaccine against C. trachomatis; however, research is ongoing. One promising candidate is a mucosal vaccine with a novel recombinant MOMP VS2/4 antigen that generates two waves of protective memory T-cells. Induction of protective immunity against genital tract C. trachomatis infection following intranasal immunization has also been proven in mice [35].
LGV experience in Poland
Up to present date, there have been only three confirmed cases of LGV in Poland. The first case in 2017 was a patient with co-infections of LGV (L2b), syphilis, and HCV [5].
In 2018, we confirmed two additional cases of C. trachomatis serovar/genovar L2b infection. The first patient was 47 years old and the second one 36 years old. Both were MSM, HIV negative, and with no coexisting STD.
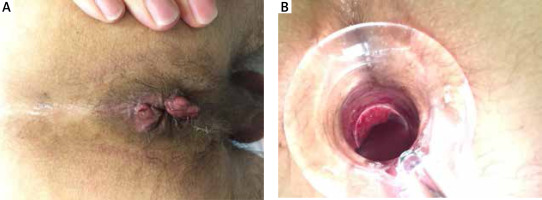
Both patients suffered from inguinal lymphadenopathy and gastrointestinal symptoms consistent with ulceration and bleeding from the lower gastrointestinal tract. The time from appearance of the symptoms to diagnosis was 1.5–2 months. After making a diagnosis of C. trachomatis infection by direct immunofluorescence, due to an LGV suspicion, swabs were taken and sent to the WHO Collaborating Centre for STIs to confirm the diagnosis. Rectal swabs from both patients contained C. trachomatis serovar/genovar L2b. Patients were also referred for anoscopy. Anoscopy in both of our patients revealed pustular proctitis with swelling bleeding and ulceration of the mucosa (Figures 1 A, B).
The first patient also underwent lymph node resection prior to the visit to our clinic due to a suspicion of cancer. He was also referred for CT scan, which showed splenomegaly. Otherwise, there were no other abnormalities in the CT scan.
Both of our patients had a history of unprotected anal sex with unknown males 2–4 weeks prior to the symptoms. Treatment with doxycycline 2 × 100 mg daily for 21 days was implemented. Both patients returned to the clinic for STD screening and had no further LGV associated complaints.
Conclusions
LGV proctitis, even if still uncommon, is increasing in incidence in many developed countries, including Poland, and sometimes can mimic other diseases like inflammatory bowel disease or cancer. Increased use of appropriate C. trachomatis NAATs for diagnosis of chlamydial infection and availability of L1-3 specific NAATs in all countries are imperative. Proper diagnosis of LGV is essential to administer appropriate treatment (3-week doxycycline regimen) and avoid severe complications and sequelae.