Introduction
Atopic dermatitis (AD; atopic eczema – AE) is a chronic, recurrent, common inflammatory skin disease, characterized by periods of exacerbation and remission. The disease manifests itself as an intensive itching accompanied by skin lesions of an eczematous morphology (acute, subacute or chronic), whose location depends on the patient’s age [1, 2].
It has been noted in recent years that in addition to comorbid atopic diseases, AD is often accompanied by a number of other non-atopic diseases, such as skin infections, cardiovascular diseases, cancer, and particularly often by mental and neurological disorders [3, 4]. A substantial number of AD patients (33–87.1% of adults and 47–80% of children) experience sleep difficulties, which exacerbate the adverse effects of the disease on their mental and socio-economic functioning [5]. The most common sleep disorders in AD patients include: difficulties with falling asleep, frequent night awakenings, problems with getting up in the morning, sleepiness during the day, sleep apnoea and even a restless legs syndrome [5–7]. Among various factors associated with sleep disorders the one which seems to be of particular importance is melatonin – a neurohormone which, among other functions, regulates the circadian rhythm of the sleep-wake cycle [8].
Aim
The aim of the study was to investigate the relationship between melatonin concentration and sleep disturbances in adult patients with severe and very severe AD.
Material and methods
The study was approved by the Bioethics Committee of the Jagiellonian University and all participants signed informed, voluntary consent to participate in the study. A group of 36 adult patients being in the exacerbation period with childhood-onset AD and 20 healthy Caucasian volunteers were qualified to the study. The characteristics of the study group are presented in Table 1. The diagnosis of the disease was established based on the Hanifin and Rajka criteria [9]; the severity of lesions was based on the Eczema Area and Severity Index (EASI, where mild AD: 1.1–7.0 points, moderate AD: 7.1–21.0 points, severe AD: 21.1–50.0 points, very severe AD: 50.1–72.0) [10]. Furthermore, each participant performed a self-assessment of the severity of the most intensive pruritus during the preceding 24 h using a Visual-Analogue Scale (VAS, where 0 points: no pruritus, 1–3: mild pruritus, 4–6: moderate pruritus, 7–8: severe pruritus, 9–10: very severe pruritus) [11]. All study participants were asked to complete the validated Polish version of the Athens Insomnia Scale (AIS) consisting of eight questions (each with three answers), which defined the following parameters of sleep during the preceding month: falling asleep, night-time awakenings, early morning awakenings, total sleep duration, quality of sleep, sense of well-being during the day, mental and physical functioning, and sleepiness during the day. The result (0–24 points) ≥ 6 points indicated sleep disorders [12].
Table 1
Characteristics of the study group
The exclusion criteria were lack of consent, age below 18 years, presence of other inflammatory, pruritic systemic diseases, taking anti-pruritic and immunosuppressive medications (up to 3 months prior to the study) and undergoing phototherapy up to 6 months prior to the study. Other causes of insomnia (psychological and internal) were excluded in all the patients. No participant had taken sleeping pills for up to 3 months prior to the study.
Three millilitres of blood for melatonin testing were taken from AD patients and from the control group. Blood was taken from the ulnar vein at 8.00 am sharp, by the same person under the same circumstances. Serum concentrations of melatonin in the blood of patients and controls were determined by ELISA, using a commercial kit from R&D System (Minneapolis, USA). Blood samples were left for 2 h at room temperature to clot, then centrifuged (3500 rpm for 10 min) and subsequently the serum samples were frozen and stored at –80°C until assayed.
Statistical analysis
Nonparametric methods were used in statistical analysis of the results: 1) Mann-Whitney U test to compare the distribution of melatonin and the severity of sleep disorders (AIS) in groups with various severity of skin lesions (EASI), 2) Spearman’s rank correlation to determine the strength and direction of the relationship between the severity of pruritus and melatonin levels. The level of statistical significance was considered to be α = 0.05. Calculations were performed by means of Dell Statistica software, version 13.
Results
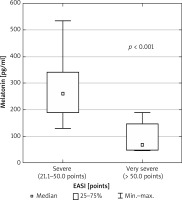
Average serum melatonin concentrations in the group of patients (median: 228.6 pg/ml; min.–max.: 46.9–534.9 pg/ml) and in the control group (median: 230.2 pg/ml; min.–max.: 159.0–538.2 pg/ml) were not statistically significantly different (p = 0.33). There were no statistical differences in serum melatonin concentrations between younger and older patients in our study. However, there was a statistically significant difference (p < 0.001) in melatonin concentration in patients with AD of different clinical severity (Figure 1). Namely, melatonin concentration in patients with very severe AD (median: 68.9 pg/ml; min.–max.: 46.9–190.5 pg/ml) was significantly lower than in patients with severe AD (median: 260.7 pg/ml; min.–max.: 129.6–534.9 pg/ml).
Figure 1
Comparison of melatonin concentration in groups of patients with severe and very severe AD (p < 0.001)
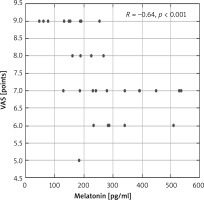
Moreover, there were significant negative correlations between the serum melatonin level and AD severity and intensity of itch assessed by VAS (R = –0.54, p < 0.001; R = –0.64, p < 0.001, respectively) (Figures 2, 3).
Figure 2
Correlation between melatonin concentration and intensity of AD (EASI points) in the study group of patients
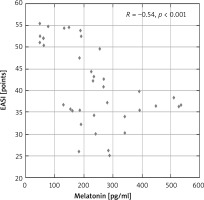
Figure 3
Correlation between melatonin concentration and pruritus (VAS points) in the study group of patients
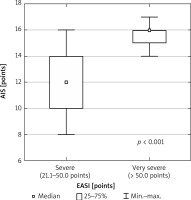
All examined patients (100%) had sleep disorders according to the AIS (> 6 pts), whereas in the control group not a single individual met the sleep disorders criteria. The median intensity of sleep disturbances in AD patients, assessed by the AIS, was 14 points (min.–max.: 8–17 pts). Sleep disturbances were statistically significantly higher (p < 0.001) in patients with very severe AD (median: 16 points; min.–max.: 14–17 pts) compared to patients with severe AD (median: 12 pts; min.–max.: 8–16 pts) (Figure 4).
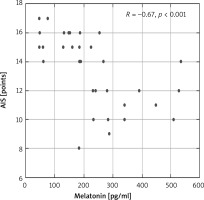
Moreover, a statistically significant negative correlation between melatonin concentration and sleep disturbances (according to AIS) was observed in patients with AD (R = –0.67, p < 0.001) (Figure 5).
Discussion
Even though AD is commonly associated with the child population, the results of an extensive study by Barbarot et al. [13] including the population of people from the USA, Japan, Canada and Europe indicate a significant underestimation of the disease prevalence in the adult population (AD symptoms were observed in as much as 8.2% of adults). To meet the needs of practising physicians, the Australian Dermatological Society decided in 2019 to develop guidelines for AD diagnosis and therapy in adults [14]. The analysis carried out by Raciborski et al. [15] demonstrated that in Poland AD was the reason for almost 260,000 specialist consultations and 8,000 hospitalisations, which translated to PLN 26.5 million spent by the National Health Fund on AD patient care. The pathogenesis of AD is associated with the interaction of environmental and immunological factors with the overlapping skin barrier deficiency; however, mechanisms related to the development of this disease are still the subject of scientific investigations [16].
Sleep is the essential functional state of the central nervous system and its disorders result in unfavourable psychosocial and economic consequences in AD patients (e.g. lower material status, concentration disorders, increased number of visits to the doctor) [17, 18].
Our observations indicate a positive correlation between the severity of skin lesions and the intensity of sleep disturbances in AD patients, which corroborates the findings of other authors, albeit obtained in research involving child patients [19, 20].
AD disturbs the physiological course of sleep in both rapid eye movement (REM) and non-rapid eye movement (NREM) phases, but the mechanisms underlying sleep disturbances in AD patients are still unexplained. The most frequently mentioned factors contributing to sleep disorders in AD patients include: itch followed by scratching, flares of comorbid atopic diseases, the so-called learned insomnia (also present in the phase of disease remission), elevated concentrations of acetylcholine, norepinephrine and histamine, which disturb the sleep-wake cycle, disorders in the population of inflammatory cells and the factors they secrete in patients’ skin, increased dysfunction of the skin barrier at night, and decreased production of melatonin [5, 8].
Melatonin (N-acetyl-5-methoxytryptamine), an L-tryptophan metabolite, was first isolated in the 1950s by dermatologist Aaron Lerner of Yale University. Although it is mostly known to be produced by a pineal gland, it is also synthesized in skin and gastrointestinal cells, lymphocytes and mast cells, among others. Melatonin is an indoleamine which, due to its amphipathic nature, easily passes through the blood-brain barrier and cell membranes, reaches the peripheral tissues (including skin) and affects the intracellular compartments [21]. It is worth noting that melatonin is produced by keratinocytes and, as an antioxidant, protects the skin against UV damage [5]. The widely recognised antioxidant properties of melatonin are associated with its anti-inflammatory effects (e.g. inhibition of induced NO synthase and cyclooxygenase-2, reduction of NF-kB and modulation of pro- and anti-inflammatory cytokines) [22]. Since melatonin is released by the pineal gland regularly in the course of the day-night cycle, it is considered to be the regulator of circadian rhythms, with its most important role being the regulation of the sleep-wake rhythm [5, 21–23].
A study by Schwarz et al. [24] from 1988 showed that in a group of 18 children with AD, the concentration of melatonin and its daily secretion rhythm were comparable to the control group (40 individuals) only in 4 children. Research of Munoz-Hoyos et al. [25] including the group of 40 children with various stages of AD indicated statistically significant differences in melatonin concentration not only between the group of patients during a flare-up and the control group (the highest melatonin concentration), but also between patients with varying severity of skin lesions. This observation is consistent with the results of our study: in the group of patients with a very severe disease, blood melatonin concentration was significantly lower than in the group of patients with severe AD.
In 2014, Chang et al. [19] having examined 72 AD patients up to the age of 18, found (based on polysomnographic and actigraphic recordings) that the severity of the disease predisposes to sleep disorders (especially in patients with SCORAD ≥ 48.7 points). A similar correlation was observed in our patients, however it should be noted that the EASI seems to be more useful for objective assessment of the severity of skin lesions when investigating sleep disorders in AD as it does not contain elements of subjective self-assessment of symptoms by the patient. Moreover, a decreased night blood melatonin concentration in patients described by Chang et al. [19] was statistically significantly associated with higher SCORAD, whereas the concentration of melatonin metabolite (6-sulfatoxymmethylatonin) in urine correlated negatively with the severity of the disease. Other factors that contributed to sleep disturbances in patients included: elevated total IgE concentration, itch and associated scratching as well as allergy to house dust mites and staphylococcal enterotoxins. Another report by the same researchers concluded that melatonin supplementation at the dose of 3 mg/day for 1 month (versus placebo) in a small group of children and adolescents proved to be effective in reducing symptoms (SCORAD; statistical significance) and time needed to fall asleep [26]. Similar conclusions were reached by Iranian authors whose research study included a group of 70 children. The researchers administered melatonin (to 35 children vs. placebo to 35 children) at a dose of 6 mg/day for 6 weeks, which resulted in a statistically significant reduction in the severity of lesions (SCORAD, objective SCORAD) and improvement in sleep parameters (Children’s Sleep Habits questionnaire) as well as in total IgE concentration. Interestingly, the treatment did not affect the severity of itching or the inflammation parameters (CRP) [27]. Results of recent studies indicate that melatonin, also due to its multidirectional anti-inflammatory and anti-apoptotic effects, may prove to be a promising therapeutic option in patients with AD [28, 29]. Our study revealed a negative correlation between the concentration of melatonin and the severity of sleep disorders and pruritus in adult patients (the lower the melatonin level, the more severe sleep disorders and pruritus). It seems worthwhile to conduct studies on the effect of melatonin supplementation on the course of the disease in adults with AD.
Considering the role of melatonin in the pathomechanism of AD, the results of experimental studies should also be mentioned, which showed that administering melatonin to mice with AD significantly reduced neuronal damage and expression of neurotoxic cytokines in the nervous system, which resulted in decreased skin itch and scratching-related damage. After melatonin administration, the IgE and CRH levels in these animals were also reduced [30].
A 2019 report published by Wroclaw-based authors investigated the impact of itching (according to VAS) on the quality of sleep (according to AIS and Pittsburgh Sleep Quality Index) in a group of 100 adult AD patients (SCORAD: 33.6 ±10.7 points; the control group consisted of 50 people). The results of that study corroborated our findings. There was a statistically significant correlation between the severity of skin lesions and the results of both insomnia scales analysis. The severity of itching was statistically significantly higher in the patient group compared to the control group. The authors suggested using other scales than SCORAD (described as a non-objective scale in the evaluation of pruritus) [31]. We heeded this advice in our study. Significant negative effects of skin pruritus on the quality of sleep (prolonged sleep onset latency, decreased sleep time and earlier awakenings) were also observed by Yu et al. [32] in an extensive survey including 5563 adult patients. Also other researchers confirmed (by means of objective methods) the impact of skin lesions severity on the quality of sleep in AD patients [33, 34].
Our study was limited by a relatively small number of patients and comparable severity of skin lesions (AD of high and very high severity). We are aware that future studies with more numerous both patients (with a wider spectrum of AD severity) and controls will be of importance. Despite this, significant differences in both groups with varying severity of the disease were revealed. Melatonin concentration was tested only once a day. Evaluating the levels of this neurohormone more frequently increases the accuracy of assays, which may be a suggestion for future studies in this area.
Conclusions
The severity of skin lesions in AD significantly affects the quality of sleep in patients. Blood melatonin concentration statistically significantly varies in patients with different degrees of skin lesions severity and correlates negatively with the severity of sleep disorders. As the results indicate a significant role of melatonin in the pathophysiology of sleep disorders in patients with AD, it would be advisable to continue the research with a larger group of patients.