Introduction
Vitiligo is a chronic skin disorder affecting 0.5–1.0% of the population, characterized by melanocyte loss. Its pathogenesis involves genetic predisposition, environmental triggers, metabolic alterations, and immune dysregulation [1]. Treatment aims to halt progression, induce repigmentation, maintain disease stability, and prevent relapses [2]. Current approaches include topical corticosteroids (TCS), calcineurin inhibitors (TCI), narrow-band UVB (NB-UVB), and laser therapies like monochromatic excimer laser (MEL) and ablative resurfacing lasers (ARLs), which enhance drug penetration and melanocyte migration [3]. Combination therapy is favoured for better efficacy, faster response, and fewer side effects [4].
Transdermal drug delivery, using dermabrasion, microneedling, and laser-assisted penetration, enhances topical treatment efficacy while avoiding systemic side effects [5]. Microneedling induces controlled microinflammation, stimulating melanocyte migration and repigmentation [6]. Compared to simple injections, microneedling devices such as dermarollers and automated needle pens offer precise depth control, reducing pain and improving tolerance [7].
While trichloroacetic acid (TCA) peels are traditionally used for depigmenting hyperpigmented skin, in vitiligo treatment [8], the controlled epidermal injury induced by TCA promotes a local inflammatory response that stimulates melanocyte migration and repigmentation in stable lesions [9].
Botulinum toxin (BTX), a neurotoxin produced by Clostridium botulinum, is widely used for cosmetic and medical treatments, including dystonia, stroke-related spasms, and Raynaud’s syndrome [10]. BTX-A blocks acetylcholine (ACh) release, causing temporary muscle paralysis [11]. The epidermis possesses an intrinsic cholinergic system involving ACh synthesis, transport, and receptors in keratinocytes and melanocytes, which is disrupted in vitiligo [12]. Elevated ACh and reduced acetylcholinesterase in vitiliginous patches normalize upon repigmentation, suggesting a role in melanogenesis regulation [13]. Microneedling enables localized BTX-A delivery into the epidermis without affecting deeper muscles, offering a safer and more precise method [14].
5-fluorouracil (5-FU), a pyrimidine analogue, inhibits DNA and RNA synthesis, promoting repigmentation in vitiligo by inducing inflammatory mediator release and metalloproteinase activation, stimulating melanocyte proliferation and migration [15].
Although previous studies, including Nofal et al. [16], have investigated microneedling combined with 5-FU or TCA, our study aimed to directly compare these two treatments under identical conditions, introduce microneedling with BTX-A as a novel group, and evaluate outcomes using comprehensive scoring systems in an Egyptian population to enhance understanding of their relative efficacy.
We hypothesized that microneedling combined with 5-FU or TCA would result in superior repigmentation outcomes compared to microneedling with BTX-A in patients with stable non-segmental vitiligo.
Aim
We aimed to evaluate and compare the efficacy and safety of combining microneedling with different topically applied medications namely 5-FU, TCA and BTX-A, for the treatment of patients with vitiligo.
Material and methods
Study design and population
This interventional comparative clinical trial was conducted on 60 patients clinically diagnosed with vitiligo. This study was approved by the ethical committee of the Benha University (approval number MS 9-4-2023). No risks were identified, and any unexpected events during the study were promptly communicated to both the patients and the ethics committee. All the records were confidential. The results of this study were used only for scientific purposes.
Eligibility criteria
Inclusion criteria
Patients were recruited consecutively from those attending the Dermatology, Venereology, and Andrology Outpatient Clinic at the Benha University Hospital between April 2023 and December 2023. All eligible patients who met the inclusion criteria and provided informed consent were enrolled until the target sample size was achieved.
Adult patients (> 18 years) with stable non-segmental vitiligo (VIDA 0), confirmed clinically and by Wood’s light, who had discontinued topical treatments for at least 2 weeks and systemic therapy or phototherapy for 4 weeks were included in the study.
Grouping
Using simple randomization with sealed, sequentially numbered opaque envelopes to ensure allocation concealment, 60 patients were randomly assigned in a 1 : 1 : 1 ratio into three groups after providing consent.
Group I (n = 20) received 5-FU (Utoral 250 mg/5 ml) at a dose of 0.1 ml/cm² following microneedling (dose was selected based on previous studies [16, 17], which demonstrated that this concentration provides effective repigmentation with a favourable safety profile when applied post-microneedling).
Group II (n = 20) received 25% TCA applied in a single uniform layer with a cotton-tipped applicator until a white frost appeared; in cases where uniform frosting was not achieved after the initial application, a second thin layer was immediately applied to ensure consistency. Approximately 20% of patients required a second layer to achieve adequate frosting.
Group III (n = 20) received BTX-A prepared with saline (20 U/ml) post-microneedling. BTX-A was reapplied every 2 weeks for six sessions, similar to the other treatment groups, to standardize treatment intervals and ensure comparability. Since BTX-A was applied topically after microneedling rather than injected, this approach aimed to maximize local modulation of the epidermal cholinergic system without inducing systemic neuromuscular effects.
Methods
All patients underwent a thorough history-taking, including age, sex, disease onset, duration, severity, aggravating factors, and previous treatment details. Clinical examination assessed vitiligo severity and stability using the Vitiligo Area Severity Index (VASI) score [18], Vitiligo Disease Activity Score (VIDA) score [19], and Physician’s Global Assessment (PGA) score [20].
Intervention
Microneedling with a dermapen (Dr. Pen Derma Pen Ultima A6®) was performed on all patients before applying different topical treatments. A thick layer of topical anaesthesia (piridocaine/lidocaine) was applied under occlusion for 30 min. Microneedling was conducted using 36-pin disposable cartridges at a depth of 0.5–1 mm and a speed of 15,000 rpm until pinpoint bleeding was observed. Haemostasis was achieved with saline-soaked gauze, and new needles were used for each session.
Following treatment, garamycin cream was applied, and patients were instructed to apply zinc oxide cream with fusidic acid cream twice daily and avoid removing crusts. Occlusion with a plastic sheet for at least 6 h was required for 5-FU and BTX-A groups. Treatments were administered at 2-week intervals for a total of six sessions over 3 months. Monthly follow-ups included standardized pre- and post-treatment photography and dermoscopic assessments.
Evaluation of treatment outcomes
Treatment efficacy was evaluated using photographic documentation, clinical examination, and standardized repigmentation scoring systems. We conducted photographic assessments at baseline, before each treatment session, and monthly for 3 months post-treatment using an iPhone 11 (8 MP camera) under standardized conditions. Clinical examination was performed at each visit, scheduled every 2 weeks, to monitor therapy progress and detect any potential side effects.
Treatment was applied to one or two selected stable vitiligo patches per patient rather than all vitiligo lesions. Although the global VASI score was used to assess overall depigmentation, this choice was made to allow standardized and validated comparisons with previous studies and to detect any possible generalized response beyond treated areas.
Repigmentation was assessed through both quantitative and qualitative methods. The VASI was used to measure the extent of depigmentation based on body surface area, with values ranging from 10% (minimal depigmentation) to 100% (complete depigmentation) [18]. Additionally, the VIDA provided a six-point scale assessing disease stability, ranging from +4 (highly active vitiligo within the past 6 weeks) to –1 (stable with spontaneous repigmentation) [19]. The PGA scale was used for qualitative evaluation, categorizing improvement into mild (0–25%), moderate (26–50%), good (51–75%), or excellent (> 75%) repigmentation [20].
Patient satisfaction
The degree of improvement according to the patient’s opinion and satisfaction: the patients were asked at the final visit about the overall satisfaction according to whether the patient was not satisfied, slightly satisfied, satisfied or very satisfied [21].
Statistical analysis
Data analysis was performed using IBM SPSS Statistics (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY,USA). Descriptive statistics included mean, standard deviation (± SD), median, and range for numerical data, while frequency and percentage were used for categorical variables. Data normality was assessed using the Shapiro-Wilk test. The Kruskal-Wallis test was applied for nonparametric comparisons across multiple groups, while the χ2 and Monte-Carlo tests analysed categorical variables. Linear regression analysis identified predictors of numerical outcomes, with the beta coefficient (β) indicating the direction and strength of associations. A p-value < 0.05 was considered statistically significant at a 95% confidence interval.
Results
There are no significant differences in sex (p = 0.921) and age distribution (p = 0.873) among the three groups. Similarly, disease onset (p = 0.743) and course (p = 0.468) were comparable. Family history (p = 0.433) also showed no significant variation across groups (Table 1).
Table 1
General characteristics of the studied groups
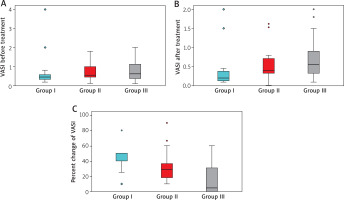
VIDA stability was observed for at least 1 year in most participants across all groups, with no significant difference before and after treatment (p = 0.071 for both). Baseline VASI scores were similar among groups (p = 0.162), but post-treatment analysis showed a significant difference (p = 0.007), with group I showing a greater improvement than group III (p = 0.029) and group II (p < 0.001). Median VASI scores decreased significantly in all groups post-treatment (p < 0.001, p < 0.001, p = 0.004, respectively). The percentage change in VASI was highest in group I (50%), followed by group II (29%) and group III (5%), with significant differences between all groups (Table 2, Figure 1).
Table 2
Comparison between the three studied groups regarding VIDA and VASI scores
| VIDA | Group I (n = 20) | Group II (n = 20) | Group III (n = 20) | Test | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| Before treatment | |||||||||
| Stable at least for 1 year | 14 | 70.0 | 10 | 50.0 | 17 | 85.0 | χ2 = 5.700 | 0.071 | |
| Activity of 6 to 12 months | 6 | 30.0 | 10 | 50.0 | 3 | 15.0 | |||
| After treatment | |||||||||
| Stable at least for 1 year | 14 | 70.0 | 10 | 50.0 | 17 | 85.0 | χ2 = 5.700 | 0.071 | |
| Activity of 6 to 12 months | 6 | 30.0 | 10 | 50.0 | 3 | 15.0 | |||
| VASI | Group I (n = 20) | Group II (n = 20) | Group III (n = 20) | Test | p1 | Pairwise | |||
| Before treatment | |||||||||
| Mean ± SD | 0.69 ±0.87 | 0.75 ±0.45 | 0.80 ±0.55 | H = 3.636 | 0.162 | – | |||
| Median | 0.45 | 0.55 | 0.62 | ||||||
| Min.–Max. | 0.18–4.0 | 0.10–1.80 | 0.10–2.0 | ||||||
| After treatment | |||||||||
| Mean ± SD | 0.38 ±0.49 | 0.54 ±0.41 | 0.71 ±0.54 | H = 9.856* | 0.007* | p2 = 0.029* p3 = 0.002* p4 = 0.389 | |||
| Median | 0.20 | 0.40 | 0.55 | ||||||
| Min.–Max. | 0.09–2.0 | 0.01–1.62 | 0.09–2.0 | ||||||
| Z | 3.936 | 3.926 | 2.842 | ||||||
| P5 | < 0.001* | < 0.001* | 0.004* | ||||||
| Change (%) | |||||||||
| Mean ± SD | 46.29 ±15.81 | 32.48 ±20.28 | 14.43 ±18.84 | H = 21.970 * | < 0.001* | p2 = 0.028* p3 < 0.001* p4 = 0.013* | |||
| Median | 50.0 | 29.0 | 5.0 | ||||||
| Min.–Max. | 10.0–80.0 | 10.0–90.0 | 0.0–60.0 | ||||||
SD – standard deviation, Min. – minimum, Max. – maximum, H – Kruskal Wallis test, Z – Wilcoxon Signed Ranks Test, P1 – comparing the three groups, P2 – comparing group I and group II, P3 – comparing group I and group III, P4 – comparing group II and group III, P5 – comparison between before and after treatment;
Figure 1
Boxplot chart for comparison between the three studied groups regarding VASI before treatment (A), after treatment (B), and regarding percent change of VASI (C)
There was a significant difference in PGA scores among the studied groups (p < 0.001), with group I showing the highest improvement (40% good, 35% moderate, 10% excellent, 10% mild). Group II had mostly moderate (60%) and mild (30%) improvement, while group III showed minimal response, with 55% having no change. Pairwise comparisons confirmed significant differences between group I and II (p = 0.021), group I and III (p < 0.001), and group II and III (p = 0.002). Patient satisfaction also varied significantly (p < 0.001), with group I having the highest satisfaction (50% very satisfied, 40% satisfied), followed by group II (45% slightly satisfied, 45% satisfied), while 75% of group III reported dissatisfaction. Pairwise comparisons confirmed significant differences between all groups (p < 0.001) (Table 3).
Table 3
Physician’s global assessment score and patient satisfaction after treatment of the studied groups
| Variable | Group I (n = 20) | Group II (n = 20) | Group III (n = 20) | Test of sig. | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| No change | 0 (0%) | 0 (0%) | 11 (55%) | χ2 = 42.845 | < 0.001* | ||||
| Mild improvement | 2 (10%) | 6 (30%) | 5 (25%) | ||||||
| Moderate improvement | 7 (35%) | 12 (60%) | 3 (15%) | ||||||
| Good improvement | 8 (40%) | 1 (5%) | 1 (5%) | ||||||
| Excellent improvement | 3 (15%) | 1 (5%) | 0 (0%) | ||||||
| P-value | P1 = 0.021*, P2 < 0.001*, P3 = 0.002* | ||||||||
| Patient satisfaction | Group I (n = 20) | Group II (n = 20) | Group III (n = 20) | Test | p1 | Pairwise | |||
| Not satisfied | 0 | 0.0 | 2 | 10.0 | 15 | 75.0 | χ2 = 54.007* | MC < 0.001* | MCp2 < 0.001* MCp3 < 0.001* MCp4 < 0.001* |
| Slightly satisfied | 2 | 10.0 | 9 | 45.0 | 5 | 25.0 | |||
| Satisfied | 8 | 40.0 | 9 | 45.0 | 0 | 0.0 | |||
| Very satisfied | 10 | 50.0 | 0 | 0.0 | 0 | 0.0 | |||
* Statistically significant when p-value < 0.05, P1 – p value between groups I and II, P2 – p-value between groups I and III, P3 – p-value between groups B and C, χ2 – chi-square, MC – Monte Carlo, P1 – comparing the three groups, P2 – comparing group I and group II, P3 – comparing group I and group III, P4 – comparing group II and group III.
The linear regression analysis identified microneedling and its combinations as the only significant predictors of clinical improvement. Other variables, including sex, age, onset, duration, baseline VIDA, and baseline VASI, showed no significant association. Among the treatment regimens, microneedling with 5-FU demonstrated the strongest positive correlation (B = 3.835), followed by microneedling with 25% TCA (B = 3.481) and microneedling with BTX-A (B = 3.362), indicating their effectiveness in enhancing clinical improvement (Table 4).
Table 4
Linear regression analysis for the predictors of percentage of clinical improvement
| Parameter | B | P-value |
|---|---|---|
| Sex | 0.004 | 0.982 |
| Age | 0.117 | 0.645 |
| Onset | 0.079 | 0.764 |
| Duration | 0.190 | 0.781 |
| Baseline VIDA | 4.494 | 0.449 |
| Baseline VASI | –7.236 | 0.116 |
| Microneedling with 5-flurouracil | 3.835 | < 0.001* |
| Microneedling with trichloroacetic acid 25% | 3.481 | < 0.001* |
| Microneedling with botulinum toxin A | 3.362 | < 0.001* |
Case presentation
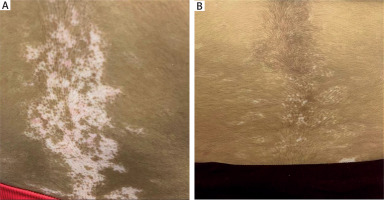
A 19-year-old female patient from group 1 underwent six treatment sessions of microneedling followed by topical application of 5-FU. Pre-treatment image shows clearly defined areas of depigmentation, while post-treatment image reveals an excellent improvement, achieving approximately 80% repigmentation observed (Figure 2).
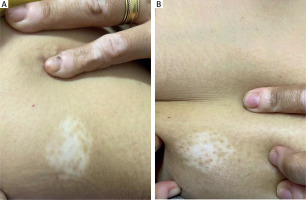
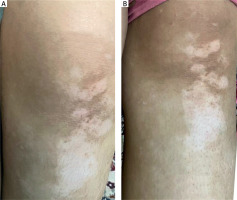
A 28-year-old female patient from group 2 underwent six treatment sessions of microneedling followed by topical application of 25% TCA. Pre-treatment image displays evident areas of depigmentation. Post-treatment image shows a moderate improvement, achieving approximately 30% repigmentation observed after the intervention (Figure 3).
A 28-year-old female patient from group 2 underwent six treatment sessions of microneedling followed by topical application of 25% TCA. The patient showed a moderate improvement, achieving approximately 30% repigmentation observed after the intervention (Figure 4).
Discussion
Combining microneedling with topically applied agents may revolutionize vitiligo treatment by accelerating response, improving outcomes, and increasing patient satisfaction. Therefore, we aimed to evaluate and compare the efficacy and safety of microneedling combined with 5-FU, TCA, and BTX-A in vitiligo treatment.
The mean age was 28.10 ±11.22 years in group I, 29.95 ±11.22 years in group II and 27.55 ±11.95 in group III with no significant differences between studies groups. This is consistent with Gaafar [22], who related that the peak age of persons with vitiligo in agreement with earlier studies documenting that vitiligo was more common below the age of 40 years.
Regarding the family history in this study, 40% of patients had a positive family history in group I, 20% in group II and 25% in group III. This finding agrees with Sharquie et al. [23], who found that 40% of patients included in their study had positive family history.
In the current study, there was a significant improvement in VASI scores among group I patients, with the majority achieving moderate to good repigmentation, while a smaller proportion showed a mild or excellent improvement highlighting the treatment’s effectiveness in enhancing repigmentation in vitiligo patients.
The study findings are consistent with Attwa et al. [17], where microneedling with 5-FU resulted in excellent to good response (> 75% repigmentation) in localized vitiligo patients. Similarly, Abdou et al. [24] reported that 40% of patients achieved > 75% repigmentation, 26.6% showed 50–75% improvement, 20% had 25–50% response, and 13.3% had < 25% repigmentation. The mechanism behind 5-FU-induced repigmentation involves the release of inflammatory mediators and metalloproteinases, promoting melanocyte proliferation and migration [15].
In the present study, there was a significant improvement in VASI scores in group I, with half of the participants achieving moderate to good repigmentation, while most others showed a mild improvement, and a small fraction exhibited excellent results indicating the treatment’s potential effectiveness in enhancing repigmentation in vitiligo patients.
This aligns with Ibrahim [25], who found that microneedling with TCA 70% led to a good to excellent improvement (> 50% repigmentation) in 53% of vitiligo patients, while 14.8% showed a moderate improvement (26–50%), and 8.2% had no effect. Additionally, Mukhtar [26] reported successful repigmentation of a lip vitiligo lesion using 100% TCA, achieving satisfactory pigmentation within 4 weeks and complete repigmentation in 6 weeks. The repigmentation mechanism of 25% TCA involves keratocoagulation, necrosis of the epidermis, exfoliation, and collagen fibre stimulation, promoting melanocyte migration [27].
This study showed that half of the participants in group III experienced no repigmentation, while 20% had a mild improvement and 30% achieved moderate to good repigmentation suggesting that BTX-A significantly improves VASI scores and may be effective in enhancing repigmentation in vitiligo patients.
To the best of our knowledge, this is the first study to explore BTX-A in vitiligo management. BTX-A blocks ACh release from presynaptic motor neurons, leading to chemical denervation and muscle paralysis [11]. The epidermis has an intrinsic cholinergic system, including ACh synthesis, transport, and degradation, which is significantly dysregulated in vitiligo patients [28]. Additionally, ACh activity is influenced by hydrogen peroxide (H2O2), with H2O2-induced oxidation of choline esterases leading to ACh accumulation in vitiligo-affected skin [29].
In the present study, 5-FU had the highest effectiveness in improving repigmentation in non-segmental vitiligo, followed by TCA, while mesobotox showed the least improvement.
TCA was significantly associated with burning sensation and perilesional hyperpigmentation compared to 5-FU, aligning with Nofal et al. [16], who reported higher side effects with microneedling combined with TCA. Similarly, Khater et al. [30] compared microneedling with 70% TCA vs. intradermal 5-FU, noting minimal side effects, with 56.2% of patients in both groups experiencing no adverse effects and no systemic side effects. Additionally, perilesional hyperpigmentation was significantly higher with 5-FU compared to mesobotox, consistent with Abdelwahab et al. [31], who identified post-inflammatory hyperpigmentation as the most common side effect, with no systemic complications reported.
This study has several limitations. The relatively small sample size and short follow-up period may limit assessment of long-term repigmentation durability and the risk of recurrence. The absence of a placebo group and objective imaging tools for repigmentation quantification could have influenced outcome evaluation. Additionally, treatment was applied only to one or two selected stable vitiligo patches per patient, while efficacy was assessed using the global VASI score, which includes both treated and untreated areas, potentially underestimating localized repigmentation effects. The lack of a microneedling-only control group further limits the ability to distinguish the effects of microneedling alone from those of combined therapies.
Moreover, despite efforts to standardize photography, variability in skin tension, positioning, alignment, scaling, and lighting may have affected the visual comparability of pre- and post-treatment images, reducing the precision and reliability of photographic assessments. Future large-scale studies with extended follow-up, direct measurement of treated areas, and standardized imaging protocols are needed to validate these findings and clarify the independent and synergistic effects of microneedling and topical treatments.