Cutaneous leiomyomas are rare benign tumours originating from smooth muscles, comprising 5% of all leiomyomas [1]. Histopathologically, they are divided into three subtypes: piloleiomyomas developed from the erector pili muscles in the pilosebaceous unit, angioleiomyomas derived from smooth muscle fibres of the cutaneous small- and medium-sized arteries and veins, and genital leiomyomas originated from vulvar, dartoic or mammary muscles [2].
Among these three subtypes, cutaneous piloleiomyomas (CPLs) are the most common. They more often develop in adults, with comparable frequency in men and women, and are frequently accompanied by pain, especially in response to friction or low temperature. CPLs may be solitary or multiple in an individual patient, and usually present as firm pink-to-red or brown papules or nodules. Multiple lesions may show disseminated, clustered or segmental (dermatomal-like) distribution [1, 2].
CPLs are usually clinically misdiagnosed and the proper diagnosis is made upon histopathological examination. Few reports characterizing the dermoscopic findings in CPLs are available in medical literature in English [3–8].
In the paper, we present 3 patients with multiple CPLs to highlight the dermoscopic findings. We also aim to summarize the diagnostic process and treatment methods of multiple CPLs.
Case 1 is a 27-year-old man referred to the Department of Dermatology with a 7-year history of multiple asymptomatic nodules of segmental distribution on the left cheek (Figure 1 A). The patient’s father had similar lesions on the left cheek and trunk. Videodermoscopy showed branching vessels and irregular yellow-white clods (Figure 1 B). Histopathological examination revealed orthokeratotic epidermis with focal intercellular oedema overlaying cords of spindle-shaped cells, which were positive for smooth muscle actin (SMA) and negative for S100 (Figures 1 C, D). On this basis, the diagnosis of segmental CPLs was made.
Figure 1
Case 1. A – Multiple pink-to-red nodules of segmental distribution on the left cheek. B – Videodermoscopy (Canfield D200EVO) showed branching vessels and irregular yellow-white clods. C – Histopathological examination revealed orthokeratotic epidermis overlying cords of spindle-shaped cells (haematoxylin & eosin). D – Positive staining for smooth muscle actin (SMA). Case 2. E – Multiple red nodules (black arrows) among psoriatic plaques; F – Videodermoscopy (Canfield D200EVO) showed discrete pigment network at the periphery, yellow-white clods, thick arborizing vessels that did not cross the midline and scattered dotted vessels. G – Histopathological examination showing cords of spindle-shaped cells (haematoxylin & eosin). H – Positive staining for desmin. Case 3. I – Two well-demarcated pink-to-red nodules in the left scapular region. J – Videodermoscopy (Canfield D200EVO) showed multifocal pigment network, white structureless areas and branching vessels. K – Histopathological examination showed cords of spindle-shaped cells (haematoxylin & eosin). L – Positive staining for caldesmon
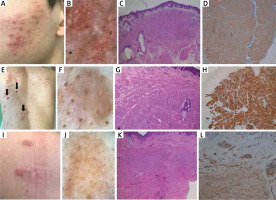
Case 2 is a 44-year-old man with multiple asymptomatic red nodules of unknown duration (Figure 1 E) and concomitant plaque psoriasis. His family history was irrelevant. Physical examination, apart from psoriatic plaques, revealed presence of over 30 pink-to-red nodules of up to 1.5 cm in diameter, located on the trunk and upper extremities. Videodermoscopy showed discrete pigment network at the periphery of all nodules and vascular structures, predominantly consisting of thick arborizing vessels that did not cross the midline of the lesion. Some bigger nodules showed polymorphic vascular pattern consisting of thick arborizing vessels at the periphery, as well as of irregularly distributed comma and dotted vessels. Yellow-white clods were observed within several nodules (Figure 1 F). Chest X-ray, as well as the ultrasound of the abdomen and peripheral lymph nodes, were within normal limits. Histopathological examination of one of the nodules was consistent with the diagnosis of piloleiomyoma: SMA (+), desmin (+), S100 (–) (Figures 1 G, H).
Case 3 is a 41-year-old woman who was admitted to the Department of Dermatology for evaluation of three asymptomatic nodules of 5-year duration. One of the lesions on the back was surgically excised 5 years prior to hospitalization (histopathological examination was not available) but recurred at the site of the surgical scar. The patient had a negative history of uterine leiomyomas and her family history was irrelevant. Three well-demarcated, dense pink-to-red nodules of 1–2 cm in diameter located in the left scapular region (two lesions) and on the left breast (one lesion) were found on physical examination (Figure 1 I). Videodermoscopy showed multifocal pigment network, white structureless areas and branching vessels (Figure 1 J). Histopathological examination was consistent with the diagnosis of piloleiomyoma (Figures 1 K, L).
Dermoscopic findings in CPLs were initially described in 2012 and included presence of discrete peripheral pigment network and central scar-like area [3]. Presence of vascular structures under dermoscopy was rarely reported in CPLs. Vessels, if present, were predominantly arborizing, while dotted, linear irregular or polymorphic vessels were observed only in single cases [5, 8]. We observed presence of multiple yellow-to-white clods in two out of 3 patients from our case series. This dermoscopic feature has so far only been reported in 1 patient with multiple segmental CPLs [7].
In the largest study to date on dermoscopic aspects of cutaneous smooth muscle neoplasms, pigment network was found to be more common in CPLs (96.5%) than in angioleiomyomas (30.8%), while vascular structures were more often observed in angioleiomyomas (69.2%) than in CPLs (15.8%) [8]. Multifocal hypopigmented areas were observed only in CPLs. Asymmetry of the tumour and presence of ulceration, multilobulated structures, atypical pigment network of multifocal distribution, vascular structures (polymorphic-atypical vessels in particular) or white structures were more commonly observed in leiomyosarcoma [8]. Dermoscopic findings in CPLs are summarized in Table 1.
Table 1
A summary of studies describing the dermoscopic features of cutaneous piloleiomyomas (CPLs)
| Parameter | Paschoal et al., 2012 [3] | Diluvio et al., 2014 [4] | Suzuki et al., 2015 [5] | Behera et al., 2018 [6] | Popadiæ et al., 2018 [7] | Zaballos et al., 2019 [8] | Current cases |
|---|---|---|---|---|---|---|---|
| Number of patients | 1 | 1 | 1 | 5 | 1 | 29 | 3 |
| Number of lesions examined | 5 | 50 | 1 | 48 | N/A | 114 | Multiple (> 50) |
| Type of examination | N/A | Polarized noncontact | N/A | Nonpolarized contact | N/A | Polarized contact | Nonpolarized contact |
| Pigment network: | 5/5 | 50/50 | 1/1 | 22/48 | Present | 110/114 | 2/3 |
| Complete regular | 5/5 | 50/50 | – | 15/22 | 98/110 | – | |
| Broken/multifocal | – | – | – | 5/22 | 4/110 | 1/3 | |
| Peripheral | – | – | – | 1/22 | 8/110 | 1/3 | |
| Blue-grey | – | – | – | 1/22 | – | ||
| Linear irregular crypts | – | – | – | 16/48 | – | – | – |
| Cerebriform pattern | – | – | – | 14/48 | – | – | – |
| Fingerprint pattern | – | – | – | 3/48 | – | – | – |
| Yellow-white area | – | – | – | 3/48 | – | – | – |
| Scar-like area | 5/5 | – | 1/1 | 1/48 | – | 8/114 | – |
| Cobble-stone pattern | – | – | – | 1/48 | – | – | – |
| Blue-grey peppering | – | – | – | 1/48 | – | – | – |
| Structureless area | – | – | – | 1/48 | – | – | 1/3 |
| Cloud-like area (interrupted irregular cotton-white area) | – | Present in some lesions | – | 5/48 | – | – | – |
| Hypopigmented areas | – | – | – | – | – | 56/114 | – |
| Whitish clods | – | – | – | – | Present (head and neck) | – | 2/3 |
| Vascular structures: | – | – | 1/1 | 5/48 | 18/114 | 3/3 | |
| Branching vessels | – | 5/5 | 11/18 | 3/3 | |||
| Dotted vessels | 1/1 | – | 4/18 | 1/3 | |||
| Polymorphic vessels | – | – | – | 2/18 | 1/3 | ||
| Circular and/or elongated hyperpigmented structures with hypopigmented centre | 4/5 | – | – | – | – | – | – |
| Hypertrichosis | – | – | 1/1 | – | – | – | – |
| Symmetry | – | – | – | – | – | 110/114 | – |
| Multilobulated | – | – | – | – | – | 1/114 | – |
| Ulceration | – | – | – | – | – | 2/114 | – |
Multiple cutaneous leiomyomas should raise the suspicion of multiple cutaneous and uterine leiomyomatosis (MCUL), an autosomal dominant condition with incomplete penetrance, formerly referred to as Reed’s syndrome [9]. In 2001, MCUL was linked to renal cancer and the term “hereditary leiomyomatosis and renal cell cancer” (HLRCC) was coined [10]. Since then, the diagnosis of HLRCC has been made in approximately 180 families worldwide [11]. Mutations of the fumarate hydratase (FH) tumour suppressor gene are suggested to be responsible for the development of multiple leiomyomas [1, 11]. The syndrome has been predominantly linked to type 2 papillary renal cell cancer (PRCCII) of an exceptionally aggressive course [12]. So far, the screening guidelines for MCUL or HLRCC syndrome have not been established. Follow-up should include annual skin and gynaecological check-up, as well as annual renal ultrasound [1].
Treatment of multiple CPLs remains challenging as surgical excision is usually not possible. Several strategies for leiomyoma-associated pain have been implemented so far. Calcium channel blockers have been predominantly used. However, data on their efficacy are contradictory [1]. Another treatment strategy is based on the destruction of nerve fibres e.g. with the ablative or fractional carbon dioxide (CO2) laser [13]. Recent reports highlight also high efficacy of botulinum toxin type A (BT-A), which reduces muscle contraction and inhibits release of pain mediators including substance P, glutamate and calcitonin gene-related peptide (CGRP) [14]. Other treatment options of leiomyoma-associated pain comprise nifedipine, nitroglycerin, gabapentin, doxazosin, lidocaine, capsaicin and phenoxybenzamine [1].
In conclusion, CPLs are rare tumours with a wide variety of dermoscopic presentations. Early diagnosis of multiple CPLs is of importance as regular assessment for leiomyosarcoma and/or renal cancer should be performed in such cases.
Conflict of interest
Magdalena Żychowska, Joanna Łudzik, Magdalena Szczepanik, Anna Józefiak, Patrycja Niewinna, Agata Drożdżyk, and Elżbieta Ostañska declare no conflict of interest.
Adam Reich has worked as a consultant or speaker for AbbVie, Bioderma, Celgene, Chema Elektromet, Eli Lilly, Galderma, Janssen, Leo Pharma, Medac, Menlo Therapeutics, Novartis, Pierre-Fabre, Sandoz, and Trevi, and participated as the Principal Investigator or a Subinvestigator in clinical trials sponsored by AbbVie, Drug Delivery Solutions Ltd, Galderma, Genentech, Janssen, Kymab Limited, Leo Pharma, Menlo Therapeutics, MetrioPharm, MSD, Novartis, Pfizer, and Trevi.







