Introduction
Allergic diseases are one of the most serious health problems worldwide. According to the latest European Academy of Allergy and Clinical Immunology (EAACI) report from 2016, it is estimated that by 2025 over 50% of all Europeans will be patients with at least one type of allergic disease [1]. The most common problem in clinical practice, apart from bronchial asthma, is allergic rhinitis (AR). AR is an inflammatory disease of the nasal mucosa, induced by an IgE-mediated reaction [2]. The costs generated by treatment, prophylaxis and diagnostic procedures as well as the deterioration in the quality of life of patients mean that AR is also an important epidemiological and social problem [3, 4]. Allergen immunotherapy (AIT) is the only disease-modifying treatment option available for patients with IgE-mediated allergic diseases [5, 6]. AIT induces immune tolerance, and prevents the development of new sensitization and the progression from AR to asthma [7, 8]. The effectiveness of immunotherapy depends on the type of sensitizing allergen. Studies show that the treatment in selected patients with intermittent allergic rhinitis is more effective [9]. The identification of specific biomarkers, which may predict response to AIT treatment in AR patients, is currently an active field of research in the aspect of recommended personalization of medicine [10, 11].
Aim
The aim of the study was to evaluate rhinological parameters that are practicable in the specialist office in patients with intermittent allergic rhinitis qualified for treatment with subcutaneous allergen immunotherapy (SCIT).
Material and methods
Study setting and participants
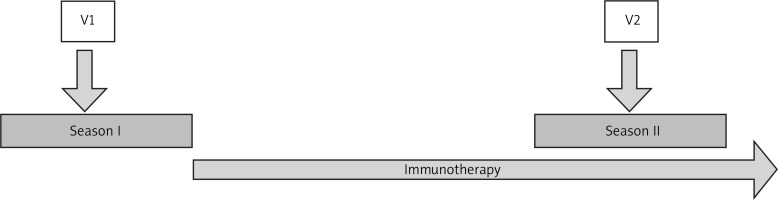
This single-center prospective observational study included patients of an allergy and ENT outpatient clinic with intermittent allergic rhinitis qualified for subcutaneous immunotherapy in accordance with the EAACI guidelines in the pollen seasons from January to July 2018 and 2019 [12]. Diagnosis of intermittent allergic rhinitis was based on clinical history and skin prick test results. Rhinitis was classified as intermittent according to ARIA criteria [13]. The patients with intermittent allergic rhinitis were evaluated twice: before AIT during the pollen season (V1) and in the next pollen season (V2) after introduction of subcutaneous immunotherapy (Figure 1). Both during the initial visit (V1) and at follow-up (V2) total nasal symptom score (TNSS), rhinomanometry and nasal cytology were performed.
Figure 1
Study flow. V1 – the first visit during the pollen season with qualification to AIT, V2 – the second visit during the next pollen season after the course of AIT
All patients denied the use of nasal or systemic corticosteroids, antihistamines or antileukotrienes for least 14 days prior to each assessment. Exclusion criteria covered: patients under 18 years of age, concomitant bronchial asthma, allergy to perennial allergens such as house dust mites, Alternaria, Cladosporium, animal dander, patients with chronic rhinosinusitis with nasal polyps (CRSwNP), clinically relevant nasal septum deviation and contraindications to SIT according to EAACI recommendation [12, 14].
Skin prick tests
The skin prick tests were performed according to the European Academy of Allergy and Clinical Immunology guidelines. Wheal diameters ≥ 3 mm were considered as positive [15]. The panel consisted of: house dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus), Alternaria tenuis, animal dander (cat, dog), grass pollen, rye, tree (birch, alder, hazel, beech) pollen, mugwort, positive (histamine 10 mg/ml) and negative (physiological saline 0.9% NaCl) controls. Allergen-specific IgE serum concentrations were not measured in the studied patients.
Severity allergic rhinitis evaluation
Total nasal symptom score (TNSS) was used to measure the clinical severity of AR and it included the following four symptoms: nasal congestion, runny nose, nasal itching and sneezing in the last 2 weeks. The symptoms were rated on a scale of 0 to 3 for each symptom, with 0 meaning no symptoms and the 3 most pronounced symptoms in the last 2 weeks. The maximum number of points was 12. The severity was assessed as mild for points 0–4, moderate for 5–8 and severe for 9–12.
Rhinomanometry
Rhinomanometry using Rhinotest MP 1000 (producer MES Poland) according to the International Committee for the Standardization of Rhinomanometry (ICSR) guidelines was performed. The values of total flow and total resistance at the 150 Pa level were calculated [16].
Nasal cytology evaluation
The nasal mucosa samples under direct vision in anterior rhinoscopy were collected. Two scrapes of the epithelial membrane of the inferior turbinate using disposable nasal brushes were performed to obtain the sample. The specimen was immediately smeared on a glass slide and fixed for 1 min in 95% ethyl alcohol and stained with hematoxylin and eosin. Slides were examined using oil immersion light microscopy. The percentage of eosinophils per 100 cells was calculated [17].
Immunotherapy
Therapy was performed with depot allergoids (Purethal-HAL Allergy B.V., Leiden) – a mixture of tree pollen, grass pollen or grass/tree pollen at a concentration of 20 000 BAU (bioequivalent allergy unit)/1 ml. Patients received a conventional administration schedule of SIT by subcutaneous injections of Purethal, starting with 0.05 ml, and then administered at weekly intervals until the maintenance dose (0.5 ml) was reached. Subsequently, maintenance dosages, corresponding to 0.5 ml of drug solution, were given at 4-weekly intervals.
Statement of ethics
The research was approved by the Bioethics Committee at the Medical University of Silesia in Katowice, Poland. Resolution No. KNW/0022/KB1/107/I/16/18. Written informed consent was obtained from each participant prior to the study.
Statistical analysis
Data are presented as median with interquartile range for variables with non-normal distribution and mean ± SD for variables with normal distribution. Wilcoxon’s test to compare non-normal variables, Kruskal-Wallis test to compare more than two groups and Spearman´s rank test to evaluate associations between variables were used. P-values < 0.05 were considered significant. Analysis was performed using Statistica 13.3 software (Statistica 13.3, StatSoft Poland).
Results
Forty-two patients (female: 19; 45%, male: 23; 55%) in the age range from 18 to 51 years with moderate (16, 38%) and severe (26, 62%) intermittent allergic rhinitis were enrolled in this study and qualified for subcutaneous immunotherapy. Fourteen (33.3%) patients were desensitized with grass pollen allergen extracts – subgroup G; 12 (28.6%) with tree pollen allergen extracts – subgroup T; and 16 (38.1%) with grass and tree pollen allergen extracts – subgroup GT. The subgroups were comparable in terms of age and sex.
The characteristics of the study groups are summarized in Table 1.
Table 1
Characteristics of all patients and subgroups sensitized to grass pollen (G), tree pollen (T), and to grass and tree pollen (GT)
All patients were followed up in the next season (V2). All examined parameters significantly improved after 1 year of allergen immunotherapy: both percentage of eosinophils in nasal mucosa samples and TNSS symptoms decreased, whereas the nasal flow rate increased and nasal resistance decreased. All results are shown in Table 2. In 26 (62%) TNSS was assessed as mild, in 14 (33%) as moderate and in 2 (5%) as severe rhinitis.
Table 2
Rhinological parameters in patients before and after one course of subcutaneous immunotherapy
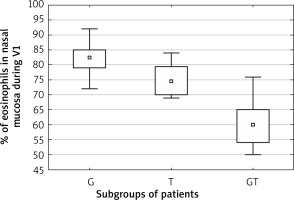
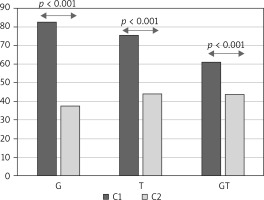
A significant correlation was found between the absolute change in TNSS and change in percentage of eosinophils in nasal mucosa assessed before beginning and after the first year of subcutaneous allergen immunotherapy (rs = 0.39, p < 0.05; Spearman correlation test). The comparisons of subgroups of patients sensitive to grass pollen, tree pollen or grass and tree pollen revealed that the change in percentage of eosinophils in nasal mucosa samples was significantly different in the subgroups and was the highest in the subgroup sensitive to grass pollen (44.5 (40–52)), less in the subgroup sensitive to tree pollen (30.5 (26–36.5)) and the least in the grass and tree pollen sensitive group (18 (31–21)), p < 0.001, Kruskal-Wallis test (Figures 2, 3).
Discussion
Ineffective pharmacological treatment in patients with intermittent allergic rhinitis may be reduced via allergen immunotherapy [5]. Allergen immunotherapy is a long-term therapeutic process and can cause some side effects; therefore qualification for this treatment should be considered very carefully [18, 19]. The new treatment strategies for SCIT require identification of factors predicting better effectiveness of this treatment [20, 21]. The efficacy of SCIT has been confirmed by numerous clinical trials and a meta-analysis [22, 23]. In this study, the authors performed a rhinological assessment in patients with intermittent allergic rhinitis during the pollen season and in the next season after initializing subcutaneous immunotherapy. In order to assess the rhinology state, the standardized methods of high diagnostic relevance, available in everyday clinical practice, were used. Objective and subjective methods were used. Among the objective methods of rhinological assessment, rhinomanometry is recommended [16]. In our study, we used Eccles’ guidelines for rhinomanometric testing [24]. After one course of SCIT, a significant increase of the air flow in rhinomanometry was observed, accompanied by a significant decline in total resistance. Rhinomanometric evaluation was confirmed by a subjective standardized test using TNSS. TNSS is recommended by the authors in order to objectively assess the severity of symptoms of intermittent allergic rhinitis [25]. The TNSS assessment showed a significant reduction in the severity symptoms.
It is recommended to find biomarkers which may predict a positive response to SCIT treatment in AR patients [26]. The ratio of specific IgE to total IgE ratio before treatment and basophil activation can be considered as a potential biomarker which can predict the efficacy of SCIT [27, 28]. For the selection of the right extracts for allergen immunotherapy composition Matricardi recommends a molecular diagnosis (Matricardi 2019). In our study we found that percentage of eosinophilic infiltration in nasal cytology can predict clinical efficacy of subcutaneous immunotherapy. The patients with high eosinophilic infiltration of the mucous membrane had markedly greater rhinitis symptoms reduction after SCIT. The reduction was particularly significant in patients allergic to grass pollen. Nasal cytology results confirm the reports suggesting that monovalent allergies to grass pollen correspond to a better response to SCIT [29]. The high percentage of nasal eosinophilia in intermittent allergic rhinitis was confirmed by other researchers [30, 31]. However, nasal cytology is a simple tool which may be considered as a predictive marker of successful subcutaneous immunotherapy. To our knowledge, there are no other data concerning the nasal cytology after SCIT. The limitation of the study was the inability to compare the results with other studies, and lack of data on pollen count in particular seasons. Another limitation was the short period of observation of the allergic rhinitis patients, made only during one course of SCIT immunotherapy. Consequently, further studies are needed to elucidate the role of percentage eosinophilic infiltration of the nasal mucous membrane after allergen immunotherapy in intermittent allergic rhinitis.
Conclusions
Objective and subjective methods of rhinological assessment confirmed high effectiveness of SCIT in intermittent allergic rhinitis. A high percentage of eosinophils in nasal cytology before subcutaneous immunotherapy can predict its clinical efficacy for allergic rhinitis, especially in grass pollen allergy.