Introduction
Neonatal lupus erythematosus (NLE) is a congenital autoimmune condition in which the transplacental passage of immunoglobulin G (IgG) directed against auto-antigens causes clinical symptoms in the foetus or neonate. Bridge and Foley first described a “maternal lupus erythematosus factor” that passes into the body of a child in 1954; in the same year, McCuistion and Schoch reported the first case of NLE cutaneous manifestation in a newborn of an asymptomatic mother who later had been diagnosed with systemic lupus erythematosus (SLE). The first description of congenital heart block (CHB) in a neonate whose mother had SLE was disclosed in 1957. Estimates indicate that NLE occurs in 0.6 per 100 000 live births annually across all populations. It is equally prevalent in males and females. Only ~2% of offspring of mothers positive for Sjogren’s syndrome autoantigen type A (Ro/SSA) or B (La/SSB) autoantibodies (aAbs) will develop the disease. However, the recurrence rate in the subsequent pregnancies rises to ~20%. Although the incidence of NLE is low, the condition bears a close clinical resemblance to other dermatoses, and hence epidemiological data may be inaccurate [1–3].
Pathogenesis
The condition is caused by the passive transfer of maternal antinuclear IgG class antibodies (Abs) across the placenta to the foetal circulation. A foetus does not produce IgG class Abs throughout its growth in utero, but only small amounts of IgM and IgA. Transplacental passage of IgG Abs from the mother to the foetus starts at the 12th gestational week when maternal blood supply to the placenta is established. Symptoms usually begin after the 16th week of gestation. ~3/4 of cases are diagnosed between the 18th and 26th week of gestation. The clinical picture of the disease is very diverse in terms of both skin and internal organ manifestations and immunological abnormalities [3, 4]. Mothers might be diagnosed with an autoimmune condition or sporadically other abnormalities before pregnancy. The most common maternal diagnosis of 755 NLE cases analysed in the recent literature review was SLE (24.4%), Sjögren’s syndrome (13.4%), and undifferentiated connective tissue disease (7%). Other diagnoses were reported less often and included mixed connective tissue disease (1.2%), rheumatoid arthritis (1.1%), leukocytoclastic vasculitis (0.4%), SLE with antiphospholipid syndrome (0.4%), antiphospholipid syndrome (0.3%), systemic sclerosis (0.1%), SLE with Sjögren’s syndrome (0.1%) and psoriasis (0.1%). 2.3% of studied mothers suffered from one or more of the following diseases: hyperthyroidism, hypothyroidism, preeclampsia, Hashimoto, autoimmune hepatitis, hepatitis B, Graves’ disease, vitiligo. No diagnosis was established before pregnancy in the ~25% of mothers, and it was not specified in the remaining ~25%. However, in 5.5% of them, the diagnosis of autoimmune disease was confirmed during pregnancy and in 29.7% in the postpartum period. Surprisingly, ~10% of mothers did not have any aAbs in their sera.
Similarly, 7.3% of NLE neonates were seronegative. Clinical pictures of these cases did not differ significantly from seropositive ones [4]. The mechanisms underlying the pathogenesis of NLE manifestations are not fully understood. The data explaining the NLE clinical symptom development is lacking. However, it is speculated that maternal aAbs bind to the autoantigens expressed on the atrioventricular (AV) node cells in the foetal heart from the 18th to the 27th week of gestation, inducing immune complex formation, fibrosis, and calcification, which characterize CHB. Still, maternal antibodies might also cross-react with calcium channels on the surface of cardiac cells and influence the electrophysiology of the developing heart [5].
Autoantibodies
Anti-Ro/SS-A, anti-La/SS-B, and to a lesser extent, anti-U1RNP Abs have the most significant impact on developing the clinical signs of NLE. The Ro/SS-A antigen consists of 2 unrelated proteins: Ro52 with a molecular weight of 52 kD localized in the nucleus and cytoplasm and the Ro60 with a molecular weight of 60 kD localized in the nucleus and nucleolus. The La/SS-B antigen is a protein present mainly in the nucleus and cytoplasm with a molecular weight of 47 kD. The anti-U1RNP antibody is a serological marker for mixed connective tissue disease (MCTD). It is directed against the 70 kD, A and C protein – essential components of the U series small nuclear RNAs involved in pre-messenger RNA processing. Although the epitope responsible for the development of NLE has not been confirmed, nor has the association between the presence of a specific antibody and the clinical course of the disease, most studies indicate a greater role of anti-Ro52 aAb as it is present in 85% of mothers whose children developed CHB [6–8]. While cardiac abnormalities are commonly linked to anti-Ro/SS-A aAb, some studies suggest that anti-La/SS-B aAb also have an association. For example, one retrospective study performed on a group of 33 women whose pregnancies were complicated by CHB identified higher concentrations of anti-La/SS-B than anti-Ro/SS-A antibodies [9].
Moreover, anti-La/SS-B aAb seems to have high specificity as it was detected only in mothers of affected children and never in mothers of healthy ones, although its sensitivity seems to be low because many offspring of anti-La/SS-B negative mothers have NLE [10]. In turn, Brito-Zeron et al. note that the majority (55%) of studied NLE neonates were positive for aAbs targeted against Ro52, Ro60, and La antigens simultaneously [11]. The results of a recent literature review are similar. Anti-Ro/SS-A was the most frequently detected aAb, found in 78.4% of mothers. However, most of them (35.5%) were positive for anti-Ro/SS-A and anti-La/SS-B aAbs. In the above study, anti-Ro/SSA aAb was present in 84.6% and anti-La/SSB aAb in 58.9% of mothers whose children developed skin lesions. The presence of maternal anti-La aAbs in the absence of anti-Ro aAbs is uncommon, and CHB cases associated with isolated anti-La aAbs make less than 1% of reported cases of autoimmune CHB. Cutaneous NLE manifestations have been identified in infants exposed exclusively to maternal anti-U1RNP aAbs. However, 1 case of CHB in a newborn exposed to anti-U1RNP antibodies in the absence of anti-Ro/SS-A/anti-La/SS-B has been described [12].
Clinical picture
The clinical characteristics of the affected children are widely diverse. Abnormalities may be observed in either an individual organ or multiple organs. The prevalence of NLE cutaneous manifestations reported in the literature is approximately 80%, hepatic dysfunction 25%, cardiac arrhythmias 20%, and haematological abnormalities 15% [8, 13]. However, in a large multiethnic cohort study which included 324 children born to anti-Ro aAb-positive mothers, the most frequently reported NLE manifestations were hepatic dysfunction (53%), cytopenias (39%), skin eruptions (21%), and CHB (13%) [14]. On the contrary, in a new meta-analysis, which looked over 755 NLE cases, the most prevalent were cardiac (65.2%), cutaneous (33.1%), haematological (15.5%), and hepatic (10,3%) manifestations. Pulmonary and neurologic complications were sporadic [4].
Skin manifestations
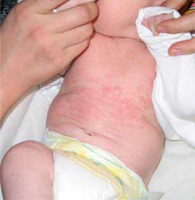
Skin manifestations of NLE are usually not noticeable directly after delivery but most commonly develop in the first month of life, typically one or 2 days after initial sun exposure. Clinically, these changes closely resemble lesions observed in the course of the annular form of subacute cutaneous lupus erythematosus (SCLE); however, although the changes may display similar characteristics, their predilection sites are different: while NLE is commonly observed on the face, neck, and scalp, this is much less the case for SCLE. The erythematous lesions exhibit features of mild inflammation, especially on the periphery. Annular or elliptic eruptions are typically around 1 cm in diameter and often blend to form larger polycyclic shapes. Papules and plaques are reported much less often. Mucosal involvement is rare and manifests as erosions on the hard palate or anogenital area [8, 15].
Photosensitivity is characteristic of NLE. Lesions usually develop in photodistribution affecting the face, neck, and scalp (95% of cases); however, changes can also be observed on the trunk and extremities (25%) (Figure 1).
UV exposure is not required as changes are present at birth in ~20% of cases, and some infants present with sun-protected intertriginous area involvement [8, 16]. In 10 % of children, lesions are disseminated all over the skin [16]. Periorbital erythema is arranged in an eye-mask shape and gives a child a “raccoon-like” appearance. The clinical presentation of the rash is quite characteristic, yet the disease often happens to be misdiagnosed, especially in neonates of asymptomatic mothers. Incorrect diagnosis results from the mistaken conclusion that dermatophyte infection, atopic dermatitis, seborrheic dermatitis, or perinatal skin injury was the cause of the lesions [17]. Skin changes of the NLE resolve spontaneously within 4 to 6 months, not later than 1 year, with the normalization of serological findings.
In most cases, no permanent changes remain; however, they may sometimes lead to mild atrophy, dyspigmentation, or telangiectasias. The lesions do not require treatment. UV protection such as sunscreens and protective clothing is obligatory in all cases. Neither topical corticosteroids nor systemic antimalarial therapy is currently advised. In the case of residual dilated superficial blood vessels, future laser treatment can be advised [18]. Granular deposits of IgG at the dermo-epidermal junction, vacuolar alteration at the interface, and adnexal structures are characteristic features of the skin biopsy histopathological examination [3].
Hepatobiliary changes
In 15–25% of cases, children with NLE display asymptomatic impairment of hepatocyte function manifested as elevations of the enzymes participating in amino acid metabolism. Occasionally, cholestasis or liver enlargement can be seen, and in exceptional cases, hepatosplenomegaly. However, these signs tend to resolve spontaneously within the first months of life and do not usually require therapy [8, 19].
Haematological changes
Haematological changes are identical to those observed in the course of SLE and manifest in about 20% of cases as the presence of thrombocytopenia (less than 100 000/μl), neutropenia (less than 1000/μl), haemolytic anaemia, or infrequently aplastic anaemia. Haematological abnormalities are usually asymptomatic, transient, and do not require treatment [8, 18]. Occasionally, these abnormalities might not be present at birth but appear later; they are usually severe in such cases, and a packed red blood cell transfusion might be necessary [20].
Both hepatobiliary and haematological abnormalities usually do not occur as isolated symptoms but accompany other clinical changes specific to NLE, most commonly cutaneous or cardiac conduction disorders.
Pulmonary changes
Transient pulmonary inflammatory abnormalities are most common [20]. Pneumonitis is rare but potentially life-threatening and regarded as the cause of death in 40% of affected children. Therefore, it is necessary to exclude an infectious aetiology in all cases, which can co-occur in newborns with NLE [21]. Treatment of immunologic pneumonitis is empirical and usually based on case reports: high-dose steroid use is recommended, sometimes combined with azathioprine, cyclophosphamide, intravenous immunoglobulins, or other immunosuppressive agents [20].
Neurological changes
Neurological changes also rarely occur in the course of NLE, and similarly to pulmonary inflammation, are not included as criteria for the classification of the disease. However, they can manifest as lenticulostriate vasculopathy with subsequent cerebral dysmaturation, cortical dysgenesis, ventriculomegaly, and dysgenesis of structures supplied by the lenticulostriate vasculature. Symptoms including seizures, strabismus, opsoclonus, truncal hypotonia, spastic paresis, myelopathies, cerebral haemorrhages, static encephalopathies, and developmental delay are usually not present at birth but have been observed in infancy [22]. Although the diagnosis seems to be serious, the abnormalities are often of a temporary and self-limiting nature [18]. Newborns of women diagnosed with SLE and positive for antiphospholipid (APL) aAbs presented an increased rate of learning disabilities and language delay [3].
Cardiac changes
Cardiac changes of the NLE mainly consist of heart block, endocardial fibroelastosis, and dilated cardiomyopathy [21, 23]. Damage is typically initiated by an immunologic reaction in which anti-Ro and, less frequently, anti-La antibodies bind to the AV node cells. The process leads to an inflammatory response and secondary fibrosis. Some reports indicate that the risk of cardiac changes increases with antibody concentration; however, cases of conduction abnormalities have been reported both in the high-titre and low-titre groups [18].
Epidemiological data show that congenital heart block is detected only in 2% of the offspring of mothers positive only for anti-Ro/SS-A aAbs and in 3% of those displaying both anti-Ro/SS-A and anti-La/SS-B aAbs [17, 18]. Symptoms are typically observed between the 18th and 24th weeks of gestation and less frequently between the 26th and 30th weeks; these include bradycardia (foetal heart rate as low as 50–70 bpm while normal ranges between 110 and 160 bpm), pericardial effusion, first-, second-, and third-degree atrioventricular blocks. Unfortunately, 78% of infants develop the complete (third-degree) block. Dilated cardiomyopathy is another manifestation of cardiac NLE. It is usually diagnosed during gestation but might also develop after birth. It can be isolated or co-occur with CHB and is invariably associated with cardiac dysfunction [7, 21]. Treatment goals are symptom control and prevention of progression and complications, including congestive heart failure, arrhythmias, sudden death, and thromboembolic events. Approximately 1/3 of DCM cases are lethal [24].
Management
As extracardiac manifestations are usually mild and of a self-limiting nature, the clinical approach to NLE is materially different than to other types of lupus. The most important recommendation is to avoid treating infants presenting exclusively with cutaneous, haematological, or hepatobiliary changes. Foetal echocardiography performed during pregnancy typically reveals a typical heart structure with no anatomical changes. Most infants do not show any signs of low cardiac output and thus do not require therapy but close monitoring. Nevertheless, CHB is associated with a high mortality rate of 18%, and pacemaker implantation is needed in ~2/3 of survivors [23]. The American Heart Association advises early detection of AV block through serial foetal echocardiography monitoring between the 16th and 28th gestational weeks with the classification of recommendations and level of evidence (COR/LOE) IIa/B and I/B. Examinations should be performed weekly or every other week in anti-Ro/LaaAbs positive women and at least weekly in mothers who have previously given birth to a child with NLE [25]. However, researchers have questioned the usefulness of repeated echocardiograms. As treatment efficacy for a first-degree block remains controversial, early diagnosis of CHB might only cause unnecessary stress in pregnant women, resulting in progression to complete block in 24 h. Therefore, foetal heart rate and rhythm monitoring (FHRM) had been proposed as an additional surveillance method.
The expectant evaluates herself using a handheld Doppler two times a day in this technique. Any anomaly she discovers is promptly verified by echocardiography. The false-positive rate was 5% in the studied group, while the false-negative rate was 0%, i.e., no women failed to detect an irregular rhythm. Further evidence is needed to confirm whether an FHRM method could become a screening tool for early CHB detection [26].
Management of CHB in utero is primarily close monitoring. When the signs of foetal distress arise, indicated preterm delivery is needed. The only intervention that improves the survival rate of affected children is permanent pacemaker implantation. Regardless, children surviving their first year of life have a good prognosis. Unfortunately, ~70% of them will require a pacemaker by the age of 10 [40].
The treatment approach to conduction abnormalities has changed significantly in recent years. Initial findings indicated potential benefits of fluorinated corticosteroids as monotherapy or in combination with other drugs [27]. Researchers recommended dexamethasone or hydroxychloroquine (HCQ) in the case of first-degree atrioventricular block. However, studies suggest that most cases resolve spontaneously rather than due to steroid use [21]. In cases of second- or third-degree block and evidence of a prenatal inflammatory process, the primary treatment method was daily administration of 4 mg of dexamethasone or 3 mg of betamethasone with or without intravenous immunoglobulins (IVIGs) throughout the remainder of the pregnancy [28, 29].
A recent meta-analysis pointed out the ineffectiveness of fluorinated steroids in lowering the neonatal pacing rate and foetal or neonatal death rate, compared to no intervention, and called attention to safety issues of such therapy. In addition to adverse effects of steroids in adults and in pregnancy, foetal adverse effects may include intrauterine growth restriction (IUGR), oligohydramnios, as well as late hypertension and diabetes [27].
In one study, 1 g/kg of body weight (BW) of IVIGs combined with prednisone was given at the 14th and 18th weeks of gestation to 8 women who previously had given birth to neonates with CHB. Seven of the 8 women delivered healthy children without CHB [30]. In another study, five NLE neonates who had a prolonged PR interval on ECG at birth, after IVIGs 1 g/kg of BW for 2 days, went back to a normal sinus rhythm [31]. Nevertheless, prophylactic IVIGs are not advised in mothers positive for aAbs and who previously gave birth to infants with cardiac manifestations unless there are confirmed conduction abnormalities regarding the current pregnancy. In addition, while their positive effect on atrioventricular conduction or mortality has not been confirmed, betamimetics are recommended for mothers in the case of the foetal heart rate falling below 55 bpm, signs of hydrops fetalis, or ventricular dysfunction [28, 29].
Prevention
The prophylactic approaches for NLE were revolutionized by the observation that pregnant women with SLE that used HCQ experienced reduced SLE activity, fewer flare-ups, and were maintained on a lower dose of steroids than those who had not used HCQ or discontinued the drug because of pregnancy. HCQ has been found to provide protective effects against cardiac NLE [32]. A retrospective case-control study confirmed a decreased risk of cardiac NLE in anti-Ro/SSA positive SLE mothers prescribed HCQ [33]. In the following cohort study, HCQ use lowered the recurrence rate of CHB by 60% [34]. Results of the subsequent smaller studies were supportive. In the analyses, exposure to HCQ remained significantly associated with a reduced risk of cardiac and cutaneous NLE manifestations [9, 35–37]. In a recent open-label prospective study, anti-Ro/SSA-positive mothers with a previous pregnancy complicated by CHB received 400 mg of HCQ a day before the end of the 10th gestational week and were followed for the primary outcome of second- or third-degree AV block. Only 4/54 evaluable pregnancies resulted in a primary outcome, a more than 50% reduction compared to the historical rate of 18% [38, 39]. Based on these findings, HCQ treatment should be prescribed to pregnant women positive for anti-Ro/SS-A/anti-La/SS-B as prophylaxis against congenital atrioventricular block [9, 32–39].
Summary
A diagnosis of NLE can be made if the mother is positive for anti-Ro/SS-A or anti-La/SS-B aAbs and the foetus or newborn presents with at least one of the following NLE manifestations: AV block, skin eruption (annular or elliptic erythema), hepatobiliary (elevation of aminotransferases, cholestasis, hepatomegaly, splenomegaly) or hematologic (anaemia, neutropenia, or thrombocytopenia), in the absence of the other plausible explanation. The histopathological examination of skin lesions is not recommended. ~50% of mothers of the affected offspring are asymptomatic and undiagnosed [4]. Therefore, screening for ANA should be offered to all women suspected of autoimmune disease before the 10–12th gestational week. In addition, 400 mg a day of HCQ should be started as soon as possible in all who tested positive for anti-Ro/SS-A/anti-La/SS-B aAbs or have a history of a child with NLE as a preventive measure for CHB. Management of CHB in utero remains controversial and there are no official guidelines. The only intervention that improves the survival rate of affected children is permanent pacemaker implantation [38, 39].