Introduction
Dupilumab is the first biologic agent used to clinically treat moderate and severe atopic dermatitis (AD) and is currently the only biologic agent used for this condition. It blocks the signalling pathways of interleukin (IL)-4 and IL-13 by binding to the IL-4 receptor chain, thus regulating the development of moderate-to-severe AD mediated by T helper (Th) 2 cells. In clinical trials, conjunctivitis, facial erythema, herpes virus infection, and injection site reactions are the most common adverse events (AE). Currently, only 1 case of facial and cervical erythema has been reported in China during treatment with dupilumab, which improved rapidly after external treatment [1]. Many studies have reported that moderate-to-severe AD was significantly improved after dupilumab injection, although head/neck dermatitis occurred with itching, flushing, and scaling. Moreover, because all the symptoms occur after dupilumab treatment, they are called “dupilumab facial redness (DFR)” [2]. However, there is currently no clear explanation for this disease’s mechanism and treatment. We reviewed and analysed the clinical data of patients with facial erythema treated with dupilumab, explored its mechanism, aetiology, and preliminary treatment plan, and provided a reference for the treatment of patients with such adverse reactions.
Aim
The study aimed to retrospectively analyse the clinical characteristics and treatment of facial erythema in patients with atopic dermatitis treated with dupilumab.
Material and methods
Patients
Patients with moderate-to-severe AD treated with dupilumab in the department of dermatology from July 2020 to May 2022 who had facial erythema or aggravated facial erythema rash during treatment were included. Inclusion criteria were as follows: patients with clinical manifestations of facial erythema accompanied or not by papules, oedema, scaling, erosive exudation, flushing, telangiectasia, and other symptoms.
This study was approved by the Ethics Committee of Ningbo No. 6 Hospital [approval number: 2022-149(L)].
Treatment plan and follow-up
Treatment plan
All patients received the first subcutaneous injection dose of 600 mg dupilumab followed by 300 mg once every 2 weeks. After 16 weeks of treatment, the clinician adjusted the treatment plan (treatment maintenance, extension of drug interval, or withdrawal) according to the patient’s disease control and increased the frequency of drug use, if necessary. Clinicians should treat patients with facial erythema based on their clinical manifestations and recent medical history.
Follow-up
Since the launch of dupilumab in 2020, our department has commenced using this drug to treat moderate and severe atopic dermatitis and some type 2 inflammatory diseases. More than 300 patients were observed and monitored by doctors and medical nurses in the outpatient department. Patients enrolled in the study were monitored for 12 months. During this period, standardized study visits were conducted in the first, fourth, eighth, and twelfth months.
Screening visits (baseline)
During the baseline visit, demographic characteristics, personal and family histories of allergic diseases, clinical manifestations, and laboratory examination results (optional) were collected. Additionally, data on disease severity (Severity Score of AD (SCORAD), Eczema Area and Severity Index (EASI), peak pruritus on a Numeric Rating Scale (NRS), and Patient-Oriented Eczema Measure (POEM)), quality of life (Dermatology Life Quality Index (DLQI) or Children’s Dermatology Life Quality Index (cDLQI)), long-term disease control (Atopic Dermatitis Control Tool (ADCT)), basic and concomitant symptoms of facial erythema, and the use of related therapeutic drugs (including dosage form, frequency, and duration) were recorded.
Follow-up visits
The follow-up visit times were as follows (V2: 1 month later, V3: 4 months later, V4: 8 months later, and V5: 12 months later). The time window was ± 2 weeks. Laboratory examination results (optional) were collected at each visit, and disease severity, long-term disease control, and treatment related to atopic dermatitis were recorded.
Unplanned visits
If necessary, unscheduled visits were conducted between regular study visits due to disease recurrence, adverse reactions, hospitalization, or changes in systemic anti-inflammatory therapy.
Efficacy evaluation
Since there is no unified disease control standard for this symptom in clinical practice, the disease control situation is evaluated according to the following standards: the reduction of facial erythema area by more than 80% compared with the baseline is defined as complete control of the disease, 50–80% as remission of the symptom, < 50% or no obvious change or aggravation of the original skin lesions as uncontrolled disease, and the occurrence of erythema area > 50% after complete control of the symptom as recurrence.
Results
Baseline characteristics
Twenty-one patients with facial erythema were enrolled, including 16 men and 5 women, aged 29.48 ±14.93 years. The duration of erythema or facial erythema aggravation was 11.67 ±13.83 weeks. The duration of continuous dupilumab use for treating moderate and severe AD was 29.86 ±27.21 weeks. The symptoms of AD in all 21 patients improved to varying degrees, and the EASI score was reduced by at least 75% at 16 weeks in 19 (90.1%) patients. Thirteen patients had a history of allergic diseases, 11 had used immunosuppressants, 3 had used new cosmetics or skin care products, 5 had a recent history of alcohol consumption, and 3 had a recent history of sun exposure. Regarding clinical characteristics, most patients had erythema, papules, and itching, while others had varying degrees of scaling, erosive exudation, oedema, flushing, telangiectasia, pain, and burning sensations. The clinical and demographic characteristics of the patients are presented in Table 1.
Table 1
Clinical features and demographic characteristics of patients (n = 21)
Treatment plan and results
In clinical practice, many uncertain factors affect the choice of treatment plans; however, clinicians mainly choose appropriate treatment plans based on clinical experience. Thus, some patients are often placed on multiple treatment plans. Among the 21 patients, 16 used conventional topical corticosteroids, topical calcineurin inhibitors, or small-molecule phosphodiesterase 4 (PDE4) inhibitors in combination with anti-allergy drugs. Nine patients’ symptoms were controlled and relieved; 4 patients’ symptoms were not relieved or became aggravated, and some had recurrent symptoms and concomitant conjunctivitis (Figure 1). Three patients had a history of sun exposure and were administered nicotinamide orally; however, the treatment effect was inadequate. Two patients were treated with oral antifungal drugs (Fluconazole) combined with external drugs (Naftifine Hydrochloride and Ketoconazole Cream), which were ineffective. After symptomatic treatment failed, 10 patients discontinued dupilumab but continued with external drugs. The symptoms of the 4 patients improved. Five patients were dissatisfied with the treatment plan and were treated with Janus kinase (JAK) inhibitors (tofacitinib 5 mg po qd or baricitinib 2 mg po qd). Facial erythema symptoms were effectively controlled, and the symptoms of moderate and severe atopic dermatitis continuously improved. The specific treatment plans and results are presented in Table 2.
Figure 1
Changes of erythema and skin lesions on the face before and after treatment with dupilumab. A – Before treatment with dupilumab, the skin around the mouth, zygomatics, eyelids, and nose was dry with a symmetrical distribution of erythema, papules with scaling, and partial lichenization. B – After 4 weeks of biologic agents combined with topical clenbuterol ointment, the erythema became pale and dark, and the area of skin lesions decreased. C – After 24 weeks of continuous use of biologic agents, the erythema on the face suddenly worsened, accompanied by local exudation, combined with conjunctivitis, and the symptoms were alleviated by topical use of eye drops (Levofloxacin Eye Drops). The face continued to be externally treated with clenbuterol ointment, and the symptoms were alleviated after 2 weeks of additional use of doxycycline 0.1 g po bid and loratadine 10 mg po qd. D – No significant erythema recurrence was observed on the face 44 weeks after the use of biologic agents
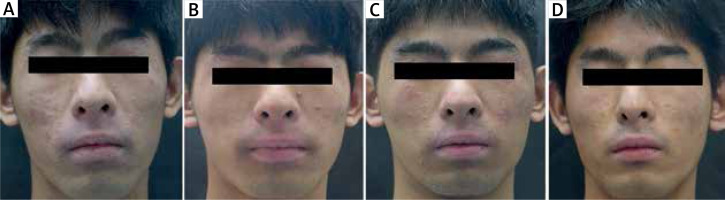
Table 2
Patients’ specific treatment plan and outcome
Follow-up
Among the 21 patients, 11 continued to receive dupilumab. After the symptoms of moderate and severe AD and facial erythema improved, the dosage was reduced to 300 mg once every 4 weeks. Simultaneously, external drug administration was maintained and then gradually reduced. After the JAK inhibitor administration, 5 patients’ blood biochemical indexes, hepatitis B III, T-SPOT, and CT, were rechecked every 3–6 months, and the drugs were gradually reduced. The other 5 patients were finally lost to follow-up.
Discussion
DFR is rarely mentioned in phase III clinical trials; however, it was the most commonly reported adverse reaction among 300 patients in our department. Based on domestic and foreign literature, we consider the following factors:
Drug allergy: In combination with this study’s findings and the views of foreign researchers, at present, DFR is not considered a hypersensitivity reaction [2], because continuous dupilumab use has not developed into a systemic hypersensitivity reaction, and the proportion of patients who improve after drug withdrawal is not high.
Treatment failure in moderate and severe AD: Researchers in other countries indicated that pathological skin biopsies of facial rash in 7 patients with DFR did not conform to the typical histopathological characteristics of atopic dermatitis; there was an increased number of dilated capillaries and infiltration of lymphoid tissue cells around the vessels in all 7 patients. In addition, there were 4 patients with epidermal hyperplasia, keratosis imperfecta, acanthosis, dermal papilla vasodilation, and large numbers of neutrophils and eosinophils around the vessels, similar to those with psoriasis. In addition, immunohistochemistry showed that the number of plasma cells, histocytes, and T lymphocytes had increased; combined with the clinical manifestations of the patients, these results demonstrated that the lesions were not typical moderate-to-severe atopic dermatitis lesions. However, the general symptoms of AD in most of the 21 patients in the present study were significantly improved; thus, this factor will not be further considered.
Activation of the Th17 pathway leads to the proliferation of Malassezia, which is mainly colonized in the sebaceous gland area. Malassezia-related seborrheic dermatitis is a possible factor often mentioned in foreign studies [3]. However, the present study demonstrated that topical and oral antifungal drugs have no obvious effects.
IL-4 receptor α blocks the regulation of helper T cell signalling, leading to contact dermatitis. In a study by Wijs et al., 7 patients with facial erythema were summarized and analysed; 3 underwent patch tests for allergic contact dermatitis (ACD), and only 1 had positive patch tests for lanolin and cocamidopropyl betaine [4]. However, allergens avoidance did not improve this erythema, and the histopathological findings did not suggest ACD. In the present study, the efficacy rate of external anti-inflammatory and oral anti-allergic drugs in such patients was only 56.2%.
Photosensitization of dupilumab: The distribution area of skin lesions and history of sun exposure suggested drug-induced photosensitivity; however, the patients did not use known photosensitive drugs and 3 patients denied the impact of ultraviolet radiation on the facial rash.
Alcoholic facial flushing: In this study, 5 patients had a long history of alcohol consumption, and 2 cases were reported abroad after 16 weeks of dupilumab treatment and the first dupilumab injection [5].
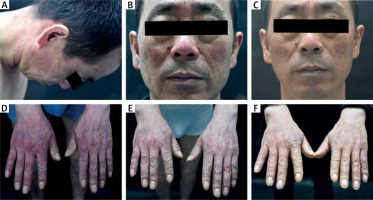
Rosacea: Increased expression of Th17 cytokines may also be conducive to the colonization of Demodex mites, causing the onset of rosacea [6]. In one of our patients, the clinical manifestation was typical rosacea combined with gloved damp erythema of both hands; however, the oral administration of hydroxychloroquine and tetracycline followed by thalidomide did not have a good effect, and the oral administration of baricitinib finally improved the symptoms (Figure 2).
Psoriasis development: Moderate-to-severe atopic dermatitis is considered a two-phase T cell-driven disease. In the acute phase, the expression of Th2 cytokines is mainly increased, whereas in the chronic phase, the expressions of Th1, Th17, and Th22 cytokines are mainly increased. Moreover, because dupilumab blocks the Th2 pathway, it may cause a change in the response dominated by Th1, Th17, and Th22, leading to a psoriasis-like response pattern in some patients [7]. JAK inhibitors, which act as blockers of multiple pathways, have shown good results in some patients.
Figure 2
Changes of facial lesions before and after treatment with dupilumab. A, B – In the 12th week after the use of biologic agents, sudden facial erythema, rosacea, and hand erythema occurred, and hydroxychloroquine tablets 0.1 g po bid, nicotinamide tablets 50 mg po tid, and loratadine tablets 10 mg po qd were ineffective after 3 weeks. C, D – Upon oral administration of thalidomide 50 mg po qn for 2 weeks at the 21st week after the use of biologic agents, the erythema flush was relieved; however, it still re-occurred repeatedly. E, F – At the 24th week, dupilumab was stopped, and baricitinib 2 mg po qd was administered orally for 1 week. Erythema on the face and hands improved, and there was no recurrence later
This study is limited by its retrospective nature and the risk of subjective bias. Therefore, a larger multi-agency study is required to further evaluate our findings.
In summary, at present, the possible causes and mechanisms of DFR are determined based on the patient’s clinical manifestations and response to treatment; therefore, the specific causes and mechanisms are unclear. In phase III clinical studies and many foreign real-world studies, conjunctivitis is the most commonly reported adverse reaction. In the present study, the most commonly reported adverse reaction was facial erythema. In total, 21 of 300 patients had DFR, while only 16 patients had ocular conjunctivitis symptoms, and 4 patients had both adverse reactions. There were no significant correlations between the two variables.
Conclusions
We observed that DFR is an underestimated AE that is not associated with the ocular adverse reactions caused by dupilumab. The inhibition of the Th2 pathway by dupilumab may cause a response dominated by Th1, Th17, and Th22, leading to the psoriasis-like response pattern that we observed. Thus, JAK inhibitors can be considered for patients with poor DFR control.








