Introduction
Off-label prescribing is defined as using medications outside conditions of the marketing authorisation including their licensed indications, dosage, age and route. Each medication’s Summary of Product Characteristics (SmPC) will by law comprise all the agreed conditions [1–3]. On the other hand, unlicensed medications are defined as medicines which do not have a marketing authorisation in the National Register of Medicinal Products. Those will include magistral drugs (specials) which formulation is modified based on the doctor’s prescription and made by the pharmacists [4]. Off-label practice was first recognized in European Directive 2010/84/EU, making the marketing authorisation holders responsible for monitoring their medicines and provide all the available information, including the ones beyond the marketing authorisation [5]. Due to legal and logistical difficulties in organizing clinical trials in paediatrics, oncology and psychiatry or in rare diseases, prescribing medications off-label in those areas is a common practice [6, 7]. Therefore, off-label prescribing is usually connected with unmet needs for therapeutics in some populations or conditions [8, 9]. It is a widely accepted concept that off-label prescribing, which practically always happens in an intended manner, needs to be made safe and researched further, also from a legal and regulatory view [8]. Therefore, more information is needed on the medications regularly prescribed outside the marketing authorisations [9]. Atopic dermatitis (AD) is the most frequent chronic inflammatory skin disease affecting up to 20% of paediatric patients [10]. In 60% of patients, it starts before the age of 1 year which is the period when majority of the systemic and topical treatments for AD will need to be used off-label [10].
Aim
The aim of the study was to investigate the frequency of off-label prescribing and the medications involved in relation to indications and age in paediatric patients hospitalized for atopic dermatitis.
Material and methods
A retrospective study of discharge letters of patients admitted to the paediatric dermatology ward in 2019 was conducted. Inclusion criteria for the evaluated patients was atopic dermatitis as the reason for admission. Gender, age, medications provided during the stay in the hospital and prescribed on discharge were analysed. Each product’s SmPC was evaluated based on data obtained from the Register of Medicinal Products Admitted to Trade in the Republic of Poland. SmPCs were checked for each medication in relation to indications (e.g., antibiotics were considered on-label if infection was stated in documentation) and age of the treated patients. The prescriptions for so called “specials” or “magistral drugs” (medicinal products prepared in a pharmacy for an individual patient in accordance with a prescription from a doctor) were excluded from the study as there are no SmPCs available for them. Also, medications used for concomitant long-term diseases, e.g. asthma or acne were not evaluated.
Results
One hundred and seventy-five children, discharge letters of 83 (47.43%) females and 92 (52.57%) males were included in the study. The age of the patients was between 2 months and 18 years (mean: 5.6 years). In the whole group of patients, systemic and topical medications in relation to atopic dermatitis were prescribed 275 and 289 times, respectively.
Systemic medications
Out of 275 prescriptions for systemic medications, the biggest group were antihistamines (120 first-generation and 72 second-generation ones), then antibiotics (32), probiotics (23), corticosteroids (14), antivirals (7) cyclosporine (6) and methotrexate (1). Ninety six medications (34.53%) were prescribed off-label in relation to the indication, with second-generation antihistamines being the most frequent ones (72), followed by antibiotics (15), corticosteroids (6), antivirals (2) and methotrexate (1). Second-generation H1 receptor antagonists prescribed off-label were bilastine (28), cetirizine (28), levocetirizine (9), fexofenadine (3) rupatadine (3) and desloratadine (3).
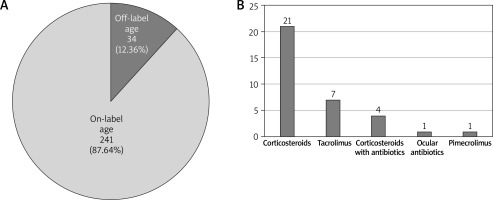
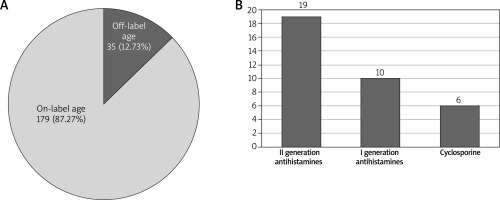
Off-label usage of medications due to the patient age took place in 35 cases (12.73%), including second-generation antihistamines (19), first-generation antihistamines (10) and cyclosporine (6).
Topical medications
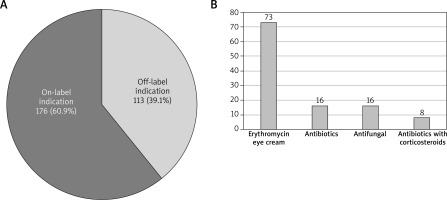
From 289 topical medications prescribed for 175 patients, 113 (39.1%) were prescribed off-label according to the indication and 34 (11.76%) to age. Erythromycin eye cream was the most frequent one (69) with no available documentation indicating infection in this area. In case of topical antibiotics, antifungal medications and antibiotics with corticosteroids (16, 16 and 8 respectively), there was not enough explanation given in discharge letters whether they were prescribed due to secondary infections of AD lesions. Thirty four topical medications which were prescribed off-label in relation to the age of the patients included corticosteroids (21), such as betamethasone (20) and alclometasone (1), then calcineurin inhibitors (tacrolimus – 7 and pimecrolimus – 1) and betamethasone with gentamycin (4) were the most frequent medications prescribed in this off-label group.
The proportion of medications prescribed off-label due to the indication and age for systemic and topical medications including the most frequent therapeutics is shown in Figures 1–4.
Figure 1
A – Systemic medications prescribed off-label versus on-label due to the indication. B – Most frequent systemic medications prescribed off-label due to the indication
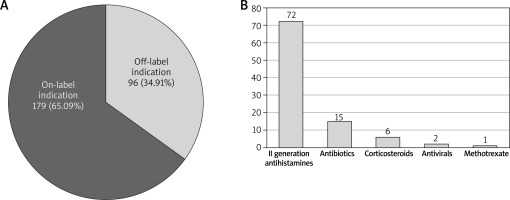
Figure 2
A – Systemic medications prescribed off-label versus on-label due to the age of patients. B – Most frequent medications prescribed off-label due to the age of patients
Discussion
The off-label practice is left for the doctor’s decision by regulatory bodies, and it is doctor’s responsibility to research the medicine, the patient’s condition and age and to obtain his or her agreement to be treated beyond the scope of the authorisation [8]. At the same time as doctors, we must use legally authorized medications first, before considering the off-label ones [8]. It is worth remembering that this process is completely different from clinical studies or research experiments. There has been so far no unified set of rules in the EU for off-label practice as it differs from country to country [7].
The results of our study showed that almost half (49.1%) of prescriptions for children with atopic dermatitis were outside SmPC marketing authorisation regarding age and/or indication, which is consistent with the literature data, making prescribing off-label in paediatric AD a frequent occurrence. The studies of prescribing in children showed that using medications off-label was present in from 13.2% up to 52% paediatric prescriptions, however in skin diseases it was reaching almost 60% [4, 11–13].
Systemic off-label medications for atopic dermatitis
The use of systemic medications in AD is usually restricted to the paediatric patients who have severe atopic dermatitis or who showed a therapeutic resistance to the conventional topical medications such as corticosteroids (TCS), calcineurin inhibitors (TCI) and phototherapy or those medications and methods are contraindicated [10, 14].
Systemic antihistamines are often used to reduce pruritus in patients with AD and to help with sleep during exacerbations of the disease, however there is no consistent evidence that they work this way [10, 15]. Moreover, they are not advised in European guidelines for AD treatment [16]. Recent studies showed a possible connection between first-generation antihistamine treatment and a higher rate of attention-deficit/hyperactivity symptoms in children with AD, therefore the authors do not recommend their long-term use [10, 17]. Gober et al. [18] showed a statistically significant association between neurodevelopmental adverse effects and use of hydroxyzine in children younger than 5 years of age. Majority of first-generation antihistamines have itch as their registered indication and in our cohort they were used in almost 44% (120) of patients. All of the off-label uses in terms of age were connected with hydroxyzine (10) in children younger than 12 months of age. Taking into account the most recent findings it seems prudent to limit the usage of first-generation antihistamines in this population. There is also no definitive evidence that second-generation antihistamines are useful as add-on therapy for AD when compared to a placebo, with only fexofenadine connected to a small improvement in pruritus assessed by patients [19]. Overall second-generation antihistamines were prescribed off-label 72 times due to the indication and 64 times due to the age of treated patients in our study. While they are very well tolerated with no significant side effects in majority of patients, it does not seem that using this treatment in AD is based on evidence.
Antibiotics (15) such as amoxicillin, cefuroxime, and rovamycine, probiotics and antiviral drugs (acyclovir – 2) could have been provided due to clinical manifestations of infections, but there was no clear explanation given in discharge letters to support that.
Oral or intravenous steroids may reduce symptoms of acute AD flares in the short term, but relapse is common when the medication is discontinued [2, 20]. Because of a possibility of significant side effects, including rebound flaring, adrenal suppression and growth retardation, use of systemic corticosteroids in children should be significantly limited [10, 21, 22]. European guidelines for treatment of AD specify that long-term use of systemic corticosteroids is not recommended and short-term use may be an option to treat acute AD exacerbations but only in most severe cases and for a limited time [16]. Guidelines also emphasise that the indication for oral steroids in children should be treated even more cautiously than in adults [10]. In our group, systemic corticosteroids were used only in 5% (14) of patients, but interestingly in 6 patients hydrocortisone sodium succinate was used off-label as neither atopic dermatitis nor inflammatory diseases reacting to systemic steroids are stated as an indication in its SmPC.
Despite that cyclosporine (CsA), methotrexate (MTX), azathioprine and mycophenolate mofetil are present in majority of guidelines for AD treatment also in children, the only systemic immunosuppressant agent which is approved in most European countries for AD is CsA. Several studies demonstrated a significant improvement in the disease activity and quality of life improvement in children treated with CsA [23–25]. Cyclosporine for atopic dermatitis indication can be prescribed for patients above 16 years of age. Furthermore, MTX remains an important option for long-term symptom control of AD with a tolerable profile of side effects, but it is not registered for atopic dermatitis [26].
In our group cyclosporine was prescribed in 6 patients, in all of them off-label as they were under 16 years of age and methotrexate was used in 1 patient, also off label. While it is not expected for marketing authorisations for “older” medications to change, more efficiency and safety data are needed especially in paediatric population. Monoclonal antibodies and JAK inhibitors are currently having their registration in AD accepted for younger patients, however their availability will be regulated separately in each country.
Topical off-label drugs for AD
Topical steroids (TCS) are the first-line treatment for AD exacerbation with minimal side effects and are used worldwide since mid-1950s [27]. It is estimated that TCS are prescribed in around of 60% doctors’ appointments for AD [20, 28]. Practically all TCS have indications specifically for atopic dermatitis or inflammatory dermatoses in SmPCs. Prescribing TCS off-label due to the age of the patients seems to be a common practice. In the USA topical desoximetasone was given off label to 52% of AD patients, followed by mometasone (30%) and betamethasone used in 18% [28]. Results regarding betamethasone are very similar to our cohort with age off-label prescriptions in 20.39%.
Topical calcineurin inhibitors, which are commonly used in AD treatment, due to the SmPC age indications are often used off-label. In the USA, 14% of yearly prescriptions for pimecrolimus and 7% for tacrolimus were prescribed off-label due to the age of the patients [29], while in our group results revealed off-label prescriptions in 20% and 25% of patients, respectively.
To summarize, we need to remember that off-label prescribing is not illegal and in many patients necessary to treat them, however the whole responsibility rests on the doctor issuing the prescription [7, 8, 30]. It is perceived as acceptable if there is no authorised alternative and it is used according to the best medical knowledge, after discussing it with the patient or/and his/her family [31]. Therefore, Lenk and Duttge [7], Bieber and Straeter [1] and recently Franca and Litewka [32] established a list of problems that doctors face when prescribing off-label and advise how to make it safe. Despite the rules of off-label prescribing being different in each country, the majority of them seem to be universal. As doctors prescribing off-label we need to:
make sure the diagnosis is right and be aware of current medical knowledge on the subject;
consider lack of compliance with previous treatments used;
carefully evaluate the indication for the off-label use;
explain in detail to the patient/parents why off-label medication is being prescribed and discuss the possible side-effects;
obtain signed informed consent form from the patient/parents and document it carefully in the patient’s notes;
discuss the decision (if needed) with the health authorities and lawyers;
investigate whether the medication can be prescribed using a subsidised prescription.
There was no information in the discharge letters on whether the off-label treatment was discussed with the patient, however the informed consent form is routinely contained in patients’ notes and therefore was not available for analysis in this study. This was due to the difficulty in obtaining “paper” patients’ notes under pandemic restrictions. Antibiotics, antifungal and antiviral prescriptions could have been related to secondary infection in atopic dermatitis patients, but there was not enough information in discharge letters given to support that.
Conclusions
Prescribing off-label in paediatric population is a common practice. Both topical and systemic medications are frequently used in AD patients off-label, mainly due to the lack of clinical trials especially with “older” therapeutics. While some of the off-label prescribed medications are unlikely to be harmful (e.g. second-generation antihistamines), others may be (first-generation ones), therefore it may be worth reconsidering their indications for atopic dermatitis. It is always prudent to document the agreement with the parents for the off-label usage.