Introduction
Urticaria is a disease with a complex pathomechanism which is characterized by the occurrence of wheals, oedema or both at the same time. Skin changes are accompanied by pruritus and/or sometimes burning sensation. Different types of urticaria have a very wide range of clinical manifestations and in one patient more than one of its types may coexist.
Confirmation of the cause in chronic urticaria seems to be a great challenge for specialists. Before selecting tests, all regional and dietary diversity as well as the incidence of infection should be taken into account. According to EAACI/GA2LEN/EDF/WAO guidelines from 2017, the first stage of diagnosis is a very detailed interview with the patient. The next step is physical examination, which should include challenge tests selected based on the interview.
Aim
The aims of the study were to assess the frequency of different types of urticaria and their coexistence in one patient, evaluate diseases associated with chronic urticaria and assess the frequency of accompanying oedema.
Material and methods
The study was divided into two parts – the retrospective one (R) and the prospective one (P). Patients from P part were divided into subgroup I and II in terms of persistence of symptoms.
Retrospective analysis included 441 chronic urticaria patients at the age of 15 or older hospitalized at the Department of Dermatology, Poznan University of Medical Sciences in 10 years. The study analysed history of the disease of all patients and then anonymised information has been placed in a specially designed form. For the prospective analysis 78 patients have been chosen out of 441 subjects previously qualified for retrospective analysis, suffering from chronic aspirin-exacerbated disease, spontaneous, autoimmune and induced urticaria. Statistical analysis was carried out in the IBM SPSS program (ver. 23).
Results
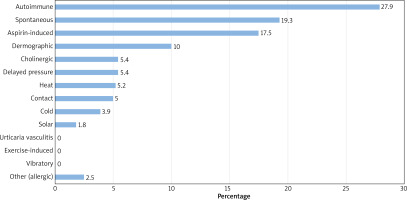
The most common type of urticaria in R part was autoimmune (27.9%), followed by spontaneous (19.3%), aspirin-induced (17.5%), dermographic urticaria (10.0%) and in P part – spontaneous (39.7%), autoimmune (30.8%), aspirin-induced (25.6%), delayed-pressure (14.1%) and dermographic urticaria (11.5%) (Figure 1).
Among the coexisting types of urticaria, the most common type was autoimmune with aspirin-induced urticaria (7.5% in R and 10.3% in P), autoimmune with dermographic urticaria (3.2% in R and 5.1% in P), autoimmune with delayed pressure urticaria (2.7% in R), aspirin-induced with dermographic urticaria (2.7% in R and 5.1% in P part), and aspirin-induced with cholinergic urticaria (2.0% in R).
We also evaluated the coexistence of different types of urticaria in 1 patient, which shows that in more than half of the patients (66.7% in R and P part) occurs only one type, in 23.3% in R and 18.7% in P – 2; in 8.5% in R and 12% in P – 3; in 1.2% in R and 2.7% in P – 4 and in only one person in R part (0.3%) – 5 types.
In over 45% of patients, the potential cause of the symptoms could not be identified. However, in 21.1% there were drugs (acetylsalicylic acid), in 12.9% – food, and in 10.9% – pressure. The evaluation in P part presented very similar results..
Most respondents from R part presented symptoms of urticaria every day (35.2%). However, patients from subgroup I (still suffering from urticaria) in P part – several times a week (30.2%).
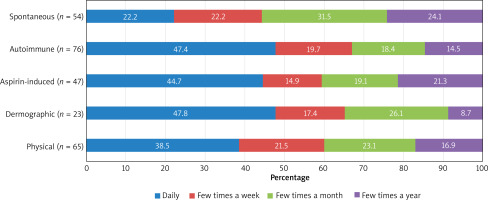
The most frequent (daily) symptoms of urticaria appeared in patients with dermographic (47.8%), autoimmune (47.4%) and aspirin-induced urticaria (44.7%) (Figure 2).
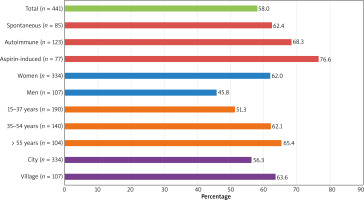
Angioedema coexisted with urticaria in over half of the patients (58% in R and 69.2% in P). It most often coexisted in patients with aspirin-induced (76.6%), autoimmune (68.3%) and spontaneous urticaria (62.4%). It occurred predominantly in women (62%) and in the > 55 years age group (65.4%), also in people living in rural areas (63.6%) (Figure 3).
In 36.7% of all patients in R part there were no additional medical conditions. The other patients, apart from urticaria, most often presented symptoms of: hypertension (19.3%), atopy (16.3%), thyroid diseases (13.6%), and chronic infections and inflammations (8.6%). The following were most commonly reported in P part: thyroid disease (19.2%), hypertension (16.7%) and atopy (11.5%).
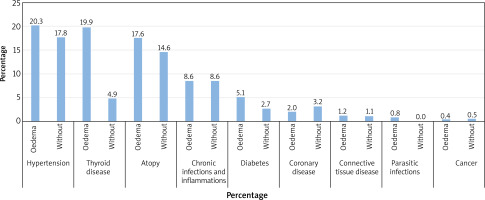
The coexistence of accompanying diseases was also evaluated in 256 patients of R part with accompanying angioedema in comparison with 185 patients without angioedema. Statistically significant differences appeared in patients with thyroid diseases (19.9% with oedema and 4.9% without oedema) (Figure 4).
Figure 4
Coexistence of comorbidities in people with accompanying angioedema (N = 256 – angioedema, N = 185 – no angioedema)
The most common medications for diseases other than urticaria used by patients in R part were antihypertensive drugs (18.6%) and hormones – including thyroid hormones – (16.8%). Other dominant drugs are drugs affecting the CNS (7.3%) and proton-pump inhibitors (5%). In P part hormones were most often used – including thyroid hormones – (24.4%), then antihypertensive drugs (16.7%), NSAIDs (12.8%), but also psychotropic drugs (7.7%).
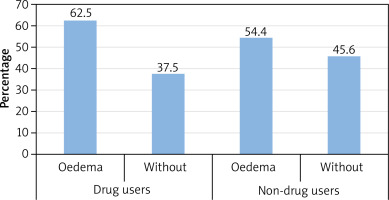
We have also performed the analysis of the coexistence of angioedema in patients of R part using medications (N = 200) and not using medications (N = 237). Four people with no information in this field were excluded from the analysis. Among drug users, there was a greater difference between the percentage of patients with or without angioedema than among non-drug users (Figure 5).
The family history of atopy and urticaria in the vast majority of respondents was negative (85.7% in R and 84.6% in P).
More than half of the patients with chronic urticaria did not undergo any procedures or operations (64.6% in R and 59% in P).
In 73% of R patients, no latent infections were detected, however 13.6% presented ENT problems, 11.5% – dental, 5.5% – problems related to inflammation in the abdominal cavity and only 2.8% – gynaecological problems.
In R patients with laryngological problems, symptoms appear most often (every day – 34.4%) and only several times a year (37.1%) – in patients with dental foci.
It was found that among the inducing factors, pressure and stress and among drugs taken for diseases other than urticaria, NSAIDs had an influence on the persistence of symptoms in P patients. There was a significant difference in the frequency of the above-mentioned factors between subgroups I and II (Table 1).
Discussion
The results of the research are similar to the available results of other epidemiological studies. All of them confirmed that spontaneous urticaria was the most common type. The Spanish authors indicated that 75% and 68% of the respondents have spontaneous urticaria [1, 2], Chinese – 68.1% [3], Brazilian and Portuguese – 25% [4], Singaporean – 89.3% [5], and Arab – 44.3% [6]. Such results confirm the thesis that in many cases it is very difficult to determine the cause of the occurrence of urticaria and it may be related to a different diagnostic approach. Most analyses do not include autoimmune and aspirin-induced urticaria in the classification.
In the R part of the study, the most common physical urticaria was dermographic (10%), then delayed pressure (5.4%) and heat urticaria (5.2%). However, the P part indicated that the most frequent urticaria was delayed pressure (14.1%), followed by dermographic (11.5%) and heat urticaria (6.4%). Both results are in line with the available publications [7–9]. Jankowska-Konsur et al. [10] pointed to dermographic urticaria (11.7%) as the most common among inducible types. Valero et al. [2] reported that the most common type among adult Spaniards was delayed pressure (22%), dermographic (21%) and solar urticaria (8%), which is most likely due to climatic differences and a high level of insolation in Spain. For comparison, in the current study, solar urticaria was one of the rarest types (1.8% in R and 1.3% in P). The diagnosis of solar urticaria is quite difficult and carries the risk of confusion with another photodermatosis. Some studies [6, 11, 12] indicated cold urticaria as a quite frequent type, however in the current study this type was placed lower in the classification (R – 3.9% and P – 5.1%).
Numerous reports emphasize the possibility of coexistence of different types of chronic urticaria. Schoepke et al. [9] reported that in 21% of all patients with dermographic urticaria, another type was also detected. Most often it was spontaneous urticaria, which is consistent with the results obtained, considering that autoimmune urticaria is a subtype of spontaneous urticaria. Cassano et al. [13] also reported a high probability of coexistence of delayed pressure urticaria with dermographic urticaria (> 50%).
In both parts of the study, in most cases it was not possible to establish a clear cause of the symptoms. Then, it was positive ASST and medications, which coincides with most common urticarias among the respondents (spontaneous, autoimmune and aspirin). In the P part, emotional stress was a very common cause, however in the R it was considered the rarest cause. Perhaps patients at home noticed a greater correlation between stressful situations and wheals than the attending physicians during a time-limited hospitalization. In many cases, no information was provided on the potential cause of the symptoms, which prompts consideration of the particular importance of the medical interview as a useful diagnostic tool in patients with chronic urticaria.
Greaves [14] mentioned hypersensitivity to ASA and autoantibodies as the most common cause of chronic urticaria. Henz et al. [15] confirmed the autoimmune background in 30% of patients, and food and drug intolerance in 25%. In a study conducted among the Chinese population, the causes in 21.6% and 4% of patients were food and drug allergies, respectively, while in as much as 72.1% the cause was temperature changes [3]. Mazur et al. [16] also pointed to food and drugs as the most frequently mentioned causes.
Autoimmune and dermographic urticaria were the most common types, which is certainly due to the difficulties in elimination of the causative agent in these types of CU, and in the autoimmune urticaria, it is virtually impossible within a certain time frame. The statistically significant results concerning the frequency of the occurrence of skin changes related to the triggering factor appeared in relation to food, medications and physical factors. However, most often the skin changes occurred in patients in whom dietary components were a potential causative factor.
Currently, it is assumed that angioedema accompanies about half of the patients with CU. The lowest coexistence was reported in Germany by Weller et al. [17] – 19% and in the Arabic study [6] – 19.7% of all patients with CU. The results below 40% were also presented in the Spanish [1] – 30.8% or Italian analysis [18] – 36%. The Chinese reported 47.3% [3], while Brazilians 50.4% [19]. Such varied results may demonstrate a lack of consistency in the diagnosis of angioedema. Most of the analyses concerning the location of the oedema are definitely consistent with the current study.
Oedema accompanied aspirin-induced urticaria more often than the other types. Perhaps this is due to the fact that aspirin may both induce and exacerbate angioedema and urticaria. Oedema definitely dominated in women and in patients at the age of > 55. Łukaszyk et al. [20] made similar observations. Angioedema also appeared more frequently in patients living in the countryside, which may be related to the exposure to different and more predisposing factors than urban ones.
Chronic urticaria is often associated with other diseases. Many researchers analysed mainly allergic diseases accompanying urticaria. Rosman et al. [21] reported that food allergy was the most common, followed by AD and allergic rhinitis. Fatani et al. [6] and Jankowska-Konsur et al. [10] also concluded that atopy is one of the most common comorbidities. Ferrer [1] reported that Spanish patients mainly suffered from autoimmune diseases, connective tissue, endocrine and myeloproliferative diseases, and only a few suffered from chronic infections.
The role of the pathogenetic importance of anti-thyroid antibodies and thyroid disease in chronic urticaria remains controversial [22]. Italian researchers in their recent epidemiological study did not notice a statistically significant relationship between the occurrence of thyroid diseases and CSU [23]. However, many authors reported a high percentage of patients with concomitant diseases of the thyroid gland [21, 24–29]. Ferrer [1] confirmed the relationship between the occurrence of chronic urticaria and autoimmune thyroid diseases. Other reported much lower rates of this coexistence, but recommended this gland to be tested during routine diagnosis [30].
In the R part the coexistence of comorbidities was also analysed in connection with the presence of angioedema. Statistically significant results concerned thyroid diseases, where 19.9% of the patients with oedema suffered from thyroid diseases, compared with 4.9% of the non-oedema patients suffering from thyroid gland diseases. Łukaszyk et al. [20] determined in their publication that the most common diseases associated with angioedema were: arterial hypertension, thyroid disease and diabetes. On the other hand, Madsen et al. [31] reported that among Danish oedema patients, the most common disease was sinusitis, HSV infection, connective tissue diseases and cystitis.
Most of the foreign publications do not present any results concerning medications taken for conditions other than urticaria which may influence the appearance of urticaria. There were only reports of exacerbations caused by taking medications [1, 3, 19]. It is worth mentioning the relatively frequent use of psychotropic drugs and drugs affecting the central nervous system by patients in this paper.
Part R also evaluated whether the use of drugs may affect the occurrence of episodes of angioedema. It can be seen that there was a much greater difference in the percentage of patients with and without oedema among drug users than among non-drug users. Other Polish authors noted that in the group of hospitalized patients with urticaria and oedema, NSAIDs and antibiotics were the most common causes of symptoms [20].
In all publications, where family history was analysed, it was exclusively related to atopy. Most of the researchers’ results are consistent with those obtained in our own analysis. Fatani et al. [6] reported that the total negative family history of patients with atopic diseases was 73.1%. Heng et al. [5] reported a negative history in 68.9%. Only the Brazilian authors noted a slight difference between positive and negative family history [19]. It turned out that almost half of the patients (44.8%) had allergic diseases in the family.
Epidemiological publications on chronic urticaria available so far do not deal with the impact of the procedures performed by the patient on the occurrence of disease symptoms. In this paper, there was no significant influence of surgery and other operations on the occurrence of the lesions.
Dental and laryngological inflammations had a significant influence on the frequency of the occurrence of symptoms. The other authors presented some discrepancies in their results. Chinese authors reported the presence of inflammations in the abdominal cavity in 10% of the population, ENT in 1.6% and urogenital in 0.7% [3]. Quite the opposite, Kacalak-Rzepka et al. [32] noted a much higher percentage (35.6%) of the presence of latent infections, including 27.8% of patients presenting hidden sources of infection in the abdominal cavity, 2.9% dental, 3.8% laryngological, and 0.9% urogenital. German researchers also identified ENT problems in almost 50% of patients [33].