Introduction
Allergy to peanut (Arachis hypogaea) affects approximately 2% of children and in most cases persists throughout adult life [1, 2]. Seventeen peanut proteins have been identified so far and registered as “Ara h” molecules by the World Health Organization (WHO)/International Union of Immunological Societies (IUIS) Allergen Nomenclature Sub-Committee [3]. Noteworthy, most of them are heat-resistant and remain conserved during food processing [4]. They belong to the major food allergens that can cause life-threatening anaphylactic reactions. Two of these molecules, Ara h 1 and Ara h 3, are the most abundant proteins in the peanut extract, together comprising at least one third of the total protein mass [5]. These storage proteins are characterized by high digestive resistance and thermostability [6] and are responsible for the greatest prevalence of IgE reactivity among peanut-sensitized patients [3].
Ara h 1 is a 7S vicilin-like globulin that comprises 12–16% of the total protein in peanut extracts and causes sensitization in about 75% of patients with peanut allergy. Ara h 3, a heat-stable 11S legumin-like globulin, constitutes about 20% of the total protein and sensitizes about 60% of patients allergic to peanuts [5, 7, 8].
Since strict avoidance of peanut-containing food is the easiest way to prevent severe allergic reactions, manufacturers must label such products [9]. However, consumers can still inadvertently be exposed to peanut allergens when foods become contaminated from processing lines shared with peanut products. Precautionary allergen labelling (PAL) such as “may contain peanuts”, “may contain traces of peanuts” or “manufactured in a setting where peanuts are processed” is voluntarily placed by food producers [10]. Nevertheless, PALs are used by the food industry inconsistently and not always reflect the actual risk to the allergic patients. Obviously, underreporting of peanut contamination can be life-threatening [11, 12], whereas an overcautious reporting may result in unnecessary fears in allergy sufferers.
Therefore, the reliable information regarding the actual content of main peanut allergens would have a great practical relevance for both patients and their doctors. Previous studies did not reveal detectable amounts of peanut in the vast majority of food products with PAL [13], however, they did not concern particular allergen components. Moreover, so far no such data are available for the Polish market.
Aim
We aimed to investigate whether food products with the label “may contain traces of peanuts”, available on the Polish market, are actually contaminated with clinically relevant amounts of two major peanut allergens – Ara h 1 and Ara h 3.
Material and methods
Thirty wrapped, shelf-stable food products with the label “may contain traces of peanuts”, two different lot numbers of each product, were purchased in Polish stores. The product selection was based on responses to questionnaires completed by children allergic to peanuts. Children indicated products that they would like to eat but have to avoid due to the possible content of peanuts. The food products were categorized as sweet snacks (n = 12), salty snacks (n = 6), candy/confectionery (n = 4), and cereal/cereal bars (n = 8). The peanut flour with a protein content of 24 g/100 g (KruKamTM, Poland) was used as a reference peanut-containing food product.
Samples of the foods were homogenized and subjected to an extraction procedure. Briefly, 0.3 g of the sample was mixed with 3 ml of the assay buffer (Indoor Biotechnologies, Inc., Charlottesville, VA, USA), incubated for 15 min at 60°C with shaking, and centrifuged at 4000 rpm for 15 min. Collected supernatants were analyzed in duplicates by using Ara h 1 ELISA 2.0 kit and Ara h 3 ELISA 2.0 kit, according to the detailed instructions of the manufacturer (both from Indoor Biotechnologies, Inc., Charlottesville, VA, USA). Based on the respective standard curve, the detection limits were 5 ng/ml for Ara h 1 and 0.5 ng/ml for Ara h 3. The experiment was performed twice, and the mean values obtained in two independent runs were used for further analysis.
Results
The concentration of Ara h 1 and Ara h 3 allergens in the peanut flour extract was high and reached approx. 700 and 400 ng/ml, respectively.
Although the absorbance of individual samples slightly varied between both runs, the pattern of Ara h allergen distribution in tested food products was similar. In general, among all extracts, prepared from both lots of 30 tested food products, the detectable amounts of Ara h 1 were found in 22 samples, whereas 18 extracts were positive for Ara h 3.
Noteworthy, 16 of 22 Ara h 1-positive samples corresponded to 8 products with detectable amounts of this allergen in both tested lots. The remaining 6 extracts positive for Ara h 1 represented 6 food products with one lot positive only.
Similarly, 14 of 18 extracts positive for Ara h 3 corresponded to 7 products with both lots being positive, while in 4 products only one sample/lot was positive.
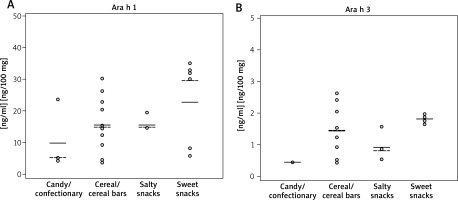
The mean concentrations of allergen components in analyzed extracts in regards to the product category were shown in Figure 1.
Figure 1
Concentrations of Ara h 1 (A) and Ara h 3 (B) in food extracts. Each dot represents a single food product contaminated with Ara h 1/Ara h 3. Mean concentrations in each group were indicated as solid lines, the median values were shown as dashed lines. The detection limits were 5 ng/ml for Ara h 1 and 0.5 ng/ml for Ara h 3
The concentrations of peanut allergens, detected in food extracts, were calculated in regards to their content in the average serving size, suggested by the manufacturers. Unexpectedly, the calculated amounts of both peanut allergens, when adjusted to the serving size of tested products, were much higher compared to those in peanut flour. In case of the latter, the serving size of 12.5 mg corresponded to the dose of 3 mg of total protein, recommended as an initial one in the oral food challenge, according to the PRACTALL consensus report [14].
Table 1 shows median amounts of Ara h 1 and/or Ara h 3 allergens in single portions of tested products.
Table 1
Ara h 1 and Ara h 3 amounts in products with detectable levels of peanut
| Product category | Ara h 1 (+) products | Ara h 3 (+) products | ||
|---|---|---|---|---|
| Median dose [μg] per 100 g of product | Median dose [μg] per serving size* | Median dose [μg] per 100 g of product | Median dose [μg] per serving size* | |
| Sweet snacks | 20 | 9.06 | 2 | 0.93 |
| Salty snacks | 15 | 3 | 1.6 | 0.48 |
| Candy/confectionery | 23 | 5.75 | 0.5 | 0.13 |
| Cereal/cereal bars | 19 | 7.99 | 1.6 | 0.52 |
| Peanut flour | 700 | 0.088 | 400 | 0.05 |
Discussion
We found that nearly one third of tested food products from all groups with precautionary allergen labelling actually contained clinically relevant amounts of peanut allergens. This finding was a bit surprising, since in previous studies less than 10% of foods with PAL were identified as contaminated with peanut traces [13]. However, most of them were tested using immunoassays with polyclonal antibodies directed against peanut extracts. The quantities of total peanut protein ranged from 0.02 up to 650 mg per 100 g of food, with median between 0.07–0.71 mg/100 g [15–19].
Noteworthy, the main limitation of polyclonal antibodies-based ELISA assays is that the measured components are not specified. Therefore, the results cannot be directly compared with those from tests of other manufacturers, which exploit different antisera or peanut extracts. However, this limitation could theoretically be overcome using monoclonal antibody-based assays to detect particular allergen molecules. By using defined allergen standards, the results of their assessment can be expressed in absolute values, i.e. allergen concentration per extract volume or per weight unit of the food product, which further may easily be compared between various studies [20]. Since the allergenic relevance of these allergen components has precisely been defined, such accurate measurements of their levels in food samples could be of great practical value.
Another critical issue in the reliable quantitation of peanut allergens in food products is the sample preparation. This is particularly important in case of highly processed foods, where the denaturation of proteins could significantly affect their solubility. Hence, due to insufficient resolubilization and incomplete extraction of the target allergen, its final concentration may be underestimated.
Furthermore, the detection efficacy may be disturbed by other food components. The well-known factor that strongly interferes with protein measurement is the presence of chocolate, mainly due to its high content of tannins and other phenolic compounds with a high protein-binding affinity [21]. Tannins may bind proteins during their extraction but may also interfere with the antibodies binding while running the ELISA.
The aforementioned interaction could explain some difficulties with protein recovery in our pilot experiments (data not shown). Therefore, although Pomés et al. suggested that the sequestering effect of chocolate tannins in Ara h 1 recovery may be reduced by the addition of non-fatty dry milk to the extraction solution [22], we decided to exclude food products with chocolate content > 50% from our study.
Among other food constituents, also large amounts of sugar or salt may interfere with the extraction process or/and ELISA procedures. Another issue could be the heterogeneous distribution of peanut traces in tested food products (e.g. only one ingredient of the filling in candies or cookies may contain peanut allergens), therefore, taking into account the above-mentioned limitations, the determination of peanut allergen components, although more precise, compared to polyclonal assays with total peanut protein measurement, should rather be considered as semi-quantitative.
While labelling the 14 main allergens, including peanuts, used as ingredients in food products is mandatory in the European Union, there is no legal definition of declaring potential contaminants. Precautionary allergen labelling (PAL) is voluntarily placed by food producers. Zuberbier et al. proposed to use the concentration of 0.5 mg of protein per 100 g of food as a threshold for voluntary declaration of allergen traces in processed food [10]. At this level, fatal reactions have never been observed, nevertheless, in a small subset of patients, allergic but not life-threatening allergic reactions can occur. On the other hand, Turner et al. suggested establishing internationally agreed “reference doses”, below which no PAL would be needed [23]. By definition, the eliciting dose 05 (ED05) is the amount of the allergen, which is predicted to provoke a reaction in 5% of at-the-risk allergic population, whereas the remaining 95% of allergic individuals would not have any objective allergic symptoms. The ED05 for peanuts was established as the amount that corresponds to 3.9 mg of total peanut protein [24]. To date, there have been no reports of fatal reactions to levels of exposure not exceeding the ED05. Nevertheless, one should still expect 2.3 episodes of anaphylaxis per 1000 exposures in the peanut-allergic population.
Noteworthy, the amount of peanut flour, used as a reference in our experimental settings, was slightly lower than ED05 and corresponded to the initial dose in the oral food challenge. Furthermore, Ara h 1 and Ara h 3 concentrations in flour extracts were much higher, compared to their levels in extracts of selected food products. However, the amounts of Ara h molecules, when adjusted to single serving sizes of tested products, in one third of them, corresponded to both allergens content in 100 mg up to 2 g of peanut flour, i.e. they exceeded several times the ED05 of the latter.
Therefore, we assume that consumption of these products may pose a significant risk for people allergic to peanuts. Obviously, each person has their individual threshold for elicitation of allergic reactions, which can be further affected by additional cofactors like exercise, alcohol, or sleep deprivation [25].
Conclusions
Taking into consideration that wrapped food products with PAL available on the Polish market indeed contain relatively high amounts of main peanut allergens, physicians should advise their patients with peanut allergy to strictly avoid such products. However, since the list of products tested in our study is the “top of the iceberg”, further studies involving a larger number of food products with PAL are necessary.








