INTRODUCTION
YKL-40, also known as chitinase-3-like protein 1 (CHI3L1) or human cartilage glycoprotein 39 (HC gp-39), is a chitinase-like glycoprotein characterized by the absence of chitinase activity due to mutations in its active site [1]. This protein is secreted by a variety of cells, including inflammatory cells such as macrophages (including tumor-associated macrophages) and neutrophils, as well as tumor cells, chondrocytes, fibroblast-like synovial cells, and vascular smooth muscle cells [2]. Although its precise biological function is not fully understood, it is believed to be involved in inflammatory responses and autoimmune diseases [3, 4]. This parameter has been exhaustively documented for the past three decades, underscoring its significance in understanding and treating these complex conditions. While YKL-40 plays significant roles in autoimmunity, previous research has primarily focused on its association with a limited number of diseases, with psoriasis being less extensively studied. Several studies suggest that YKL-40 could serve as a marker for disease diagnosis, prognosis, activity, and severity. Its role in the response to disease treatment has also been demonstrated [5]. To better understand its relationship with psoriasis, the authors analyzed 13 studies investigating the association between YKL-40 and the disease.
OBJECTIVE
The aim of the study was to investigate the role of YKL-40 in psoriasis by reviewing its association with disease diagnosis, prognosis, activity, severity, and treatment response, while identifying gaps in current research to further clarify its role in autoimmunity. This review is an important preliminary step in evaluating the feasibility of further research on this biomarker, including possible comparisons with other psoriasis markers, as well as its potential diagnostic and therapeutic applications.
METHODS
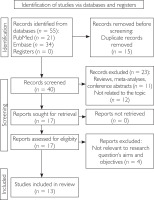
The systematic review was conducted in accordance with PRISMA guidelines (fig. 1) and included all types of articles published from database inception to January 2025, with the most recent publication dated August 23, 2023. The authors performed a literature search in PubMed and Embase using the MeSH and Emtree terms “Chitinase-3-Like Protein 1” and “Psoriasis". After eliminating duplicates, the articles were manually reviewed to ensure they met the inclusion criteria, excluding studies that did not show a connection with the cutaneous manifestations of the disease. Only original studies that had at least an abstract published in English were included in the final analysis. Mendeley Reference Manager (Version 2.134.0) was used to remove duplicates that appeared due to overlapping search terms.
RESULTS
The results of the studies were compiled based on the type of study and outcomes, including: 1) serum levels of YKL-40, 2) the association between serum levels of YKL-40 and disease severity as measured by the Psoriasis Area and Severity Index (PASI score), 3) changes in serum levels of YKL-40 following narrowband ultraviolet B (NB-UVB) treatment, methotrexate (MTX) therapy and biological treatment, 4) the correlation of serum levels of YKL-40 with other parameters, such as age, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cells (WBC), neutrophils, body mass index (BMI), glucose, lipids, and disease duration. Analyzed issues are shown in table 1.
Table 1
Summary of literature on the relationship between psoriasis and YKL-40 (chitinase-3-like protein 1, human cartilage glycoprotein 39)
| No. of study | Year | Author | Study and population | Results |
|---|---|---|---|---|
| 1 | 2011 | Imai et al. [21] | Case-control 62 psoriasis patients and 21 healthy controls Serum YKL-40 measured using ELISA Anti-YKL-40 antibody was used to assess YKL-40 expression in skin biopsy samples. | Serum levels of YKL-40 were higher in patients with psoriasis, including both PV and GPP. The levels of YKL-40 in the GPP patients were significantly higher than in the PV patients. In patients with generalized pustular psoriasis, YKL-40 levels showed correlations with inflammatory markers, including white blood cell, neutrophil, and CRP levels. Elevated levels of YKL-40 in psoriasis may be an indication of the involvement of arthritis or more systemic or severe inflammatory conditions. |
| 2 | 2013 | Jensen et al. [14] | Case-control 90 patients divided into two groups: 48 with plaque psoriasis and 42 with PsA Control group of 3,130 healthy individuals aged 21 to 84 years The levels of YKL-40 in plasma, hs-CRP, and psoriasis severity using the PASI index were studied. YKL-40 and hs-CRP levels were measured in 42 PsA patients at the start of the study and throughout 48 weeks of adalimumab treatment. In a subgroup of 14 plaque psoriasis patients starting methotrexate, YKL-40 and hs-CRP levels were measured again after 4–6 weeks. | Median plasma levels of YKL-40 were markedly higher in patients with PsA than in patients with plaque psoriasis. In patients with plaque psoriasis, there was no correlation between YKL-40 levels and disease severity (PASI). YKL-40 levels did not decrease after 4-6 weeks of methotrexate treatment. Plasma levels of YKL-40 in patients with PsA who responded to adalimumab treatment decreased over 48 weeks of treatment. |
| 3 | 2013 | Imai et al. [15] | Case-control 18 PsA patients and 29 healthy volunteers YKL-40 serum levels were measured by immunoenzymatic ELISA. | YKL-40 levels were significantly higher in PsA patients than in healthy individuals. Serum YKL-40 levels decreased significantly with improvement in PASI score after infliximab treatment. No correlation was found between YKL-40 levels and the severity of plaque psoriasis. |
| 4 | 2015 | Ahmed et al. [22] | Case-control 48 psoriasis patients and 30 healthy controls YKL-40 serum levels were measured using ELISA. | YKL-40 levels were considerably elevated in patients with psoriasis, including those with and without PsA, when compared to healthy controls. Multiple regression analysis showed that PASI and CPDAI were the most important factors influencing YKL-40 levels. |
| 5 | 2015 | Erfan et al. [16] | Case-control 127 patients above the age of 18 with active, first diagnosed, untreated, moderate or severe plaque psoriasis according to the PASI Control group – 30 healthy volunteers | Psoriasis patients had higher YKL-40 and CRP levels than controls. YKL-40 was higher in patients over 40 years than under 40, with no age-related differences in controls. No correlation was found between YKL-40 levels and disease duration. Psoriatic patients with ED had higher YKL-40 levels than controls with ED, with no differences in those without ED. YKL-40 was elevated only in RP patients. YKL-40 levels were unaffected by smoking or BMI. |
| 6 | 2017 | Salomon et al. [17] | Case-control 55 patients suffering from psoriasis The study group consisted of 21 women and 34 men, aged from 18 to 88 years 37 healthy individuals YKL-40 serum levels were measured in patients with psoriasis and controls. Inflammatory markers: CRP, ESR, WBC, neutrophil count were also measured. | YKL-40 serum concentration was significantly higher in patients with psoriasis, compared to the control group. No significant correlations were found between serum YKL-40 levels and other clinical or laboratory parameters, such as severity of skin changes, age, gender, CRP, ESR, WBC, or neutrophil count. |
| 7 | 2018 | Salomon et al. [18] | Case-control 42 patients with PsA: 28 men and 14 women, aged from 24 to 71 years 7 healthy volunteers Serum YKL-40 levels were measured using the ELISA test. Inflammatory markers, including CRP, ESR, WBC and neutrophil count were also measured. | Psoriasis patients had significantly higher YKL-40 levels than controls. No significant correlation was observed between PASI scores and YKL-40 levels before or after treatment. A moderate positive correlation was found between YKL-40 and disease duration before treatment. YKL-40 levels were not correlated with age or sex before or after treatment. |
| 8 | 2018 | Baran et al. [19] | A prospective case-control study 37 individuals with active plaque-type psoriasis and 15 sex-, age- and BMI-matched healthy volunteers were enrolled. | Median YKL-40 serum levels were significantly higher in psoriatic patients compared to the control group. No significant correlations were found between YKL-40 levels and BMI, glucose or lipid levels. No correlation was found between YKL-40 levels and PASI scores. Despite clinical improvement, serum YKL-40 levels remained unchanged following topical treatment. |
| 9 | 2019 | El-Hamd et al. [20] | Cross-sectional case-control 30 patients with PV and 10 healthy individuals Expression of YKL-40 in the epidermis and dermis of psoriasis patients The correlation between YKL-40 expression and PASI were studied. | The serum YKL-40 levels were significantly higher in patients with PV compared to healthy control subjects. After 3 months of NB-UVB phototherapy, there was a significant reduction in both serum YKL-40 levels and PASI scores in patients with PV. This study further demonstrated that median YKL-40 serum levels were significantly elevated in psoriatic patients compared to healthy controls. Additionally, median YKL-40 serum levels in patients with PV were significantly reduced following NB-UVB phototherapy compared to pre-treatment levels. |
| 10 | 2019 | Agamia et al. [11] | Case-control 30 chronic plaque psoriasis patients and 15 healthy controls | YKL-40 may be a useful inflammatory marker in psoriasis. Patients with psoriasis had significantly higher serum levels of YKL-40 compared to healthy controls. |
| 11 | 2020 | Shaheen et al. [12] | Prospective clinical-control study 30 Egyptian patients with PV and 30 healthy controls. | Significantly elevated levels of YKL-40 in both serum and tissue in patients with psoriasis compared to controls. Strong correlation between YKL-40 levels and PASI score, suggesting that higher YKL-40 levels are associated with greater psoriasis severity. No statistical correlation between YKL-40 levels and age, BMI or disease duration. |
| 12 | 2021 | Khashaba et al. [23] | Prospective cohort study Correlation of YKL-40 before and after NB-UVB phototherapy 28 patients with moderate-to-severe plaque psoriasis (plaque psoriasis) | Positive correlation between serum YKL-40 levels and psoriasis severity, as measured by the PASI index, both before and after NB-UVB treatment. The higher the level of YKL-40, the more severe the disease. Marker levels decreased after NB-UVB treatment. |
| 13 | 2023 | Elziaty et al. [13] | Clinical observational study with a therapeutic intervention 40 patients with PV with or without PsA, treated with methotrexate | Serum YKL-40 levels were significantly higher in patients with PsA compared to those with psoriasis alone. Methotrexate treatment led to a significant reduction in YKL-40 levels in both groups: psoriasis patients with and without PsA. A positive correlation was found between serum YKL-40 levels and PASI scores. |
[i] YKL-40 – chitinase-3-like protein 1/human cartilage glycoprotein 39, PV – psoriasis vulgaris, GPP – generalized pustular psoriasis, PsA – psoriatic arthritis, ELISA – enzyme-linked immunosorbent assay, PASI – Psoriasis Area and Severity Index, CPDAI – Clinical Disease Activity Index, CRP – C-reactive protein, hs-CRP – high-sensitivity C-reactive protein, ESR – erythrocyte sedimentation rate, WBC – white blood cell count, ED – endothelial dysfunction, BMI – body mass index, NB-UVB – narrowband ultraviolet B, RP – risk positive (used in the context of cardiovascular risk factors).
DISCUSSION
YKL-40 has emerged as a protein of interest in various autoimmune and inflammatory conditions due to its involvement in processes such as immune regulation, tissue remodeling, and inflammation. Despite its established role in several autoimmune diseases, its connection with psoriasis remains relatively underexplored. Given that psoriasis is a chronic inflammatory disease driven by complex immune dysregulation, investigating potential biomarkers such as YKL-40 is of particular clinical relevance.
YKL-40 modulates the immune response by enhancing the expression and release of key proinflammatory cytokines, including interleukin-6 (IL-6), interleukin-18 (IL-18) and tumor necrosis factor α (TNF-α). This modulation occurs through the activation of signaling pathways such as NF-κB and mitogen-activated protein kinase (MAPK), which are critical for cytokine transcription and amplification of inflammatory signaling [5]. In macrophages and monocytes, YKL-40 upregulates IL-6 and TNF-α expression, sustaining chronic inflammation and promoting a Th1/Th17-skewed immune profile. Additionally, YKL-40 can induce IL-18 expression, a cytokine that further enhances the production of interferon-γ (IFN-γ), perpetuating autoimmunity [6]. These effects underscore YKL-40’s role as an upstream amplifier of inflammatory cascades in autoimmune and chronic inflammatory diseases. Despite these promising findings, the biological function of YKL-40 in the context of psoriasis remains insufficiently clarified. YKL-40 is known to be involved in processes such as extracellular matrix remodeling, angiogenesis, leukocyte recruitment, and fibroblast activation, thereby contributing to the regulation of inflammatory responses [7, 8]. These mechanisms are fundamental to the pathogenesis of psoriasis and the formation of psoriatic plaques [9]. Its known ability to bind chitin-like carbohydrates and influence tissue remodeling implicates it in the abnormal epidermal proliferation and thickening characteristic of the disease [10]. The expression of YKL-40 in macrophages, neutrophils, and chondrocytes within inflammatory microenvironments suggests its potential role in the disease pathogenesis, contributing not only to cutaneous but also systemic manifestations and joint involvement. Although some studies suggest that YKL-40 may reflect general inflammation or endothelial damage, it is still unclear whether it merely serves as an indicator of these processes or actually contributes to the development of psoriasis [3].
The available literature provides only limited evidence regarding how circulating YKL-40 levels differ in patients with psoriasis. Even fewer studies explore the correlation between these levels and disease severity, as assessed by the PASI scale. The extant literature on the subject is inconclusive. Some studies have indicated a positive correlation between these parameters [11–13], but others have not confirmed such a relationship [14–20]. Discrepancies in the literature may be partly attributed to the clinical subtype of psoriasis being studied – namely, plaque vulgaris (PV), generalized pustular psoriasis (GPP), and psoriatic arthritis (PsA). Although psoriasis manifests in various forms, available data are largely confined to these three subtypes. In addition to disease subtype, inconsistencies may also arise from a tendency to generalize across disease severities. Notably, studies involving patients with moderate-to-severe psoriasis or generalized disease forms more often demonstrate a clearer correlation between YKL-40 levels and PASI scores. This suggests that the association between YKL-40 and clinical severity may be more evident in patients with extensive systemic or cutaneous inflammation, while it may remain obscure in milder or localized presentations.
Studies comparing serum YKL-40 levels in patients with moderate (PASI 5–10) or severe (PASI > 10) psoriasis vulgaris to healthy controls demonstrated a statistically significant elevation of serum YKL-40 in the PV group [16, 20]. In a study conducted by Baran et al., serum YKL-40 levels in patients with active PV were also nearly 2.5 times higher than in the control group [19]. Despite this marked elevation, none of the studies involving PV patients demonstrated a statistically significant correlation between YKL-40 levels and disease severity as assessed by the PASI score [16], even when stratified by clinical subgroups of mild, moderate, and severe disease [19]. One possible explanation for this lack of association is the exclusive focus on serum YKL-40, without parallel analysis of tissue-specific expression. This represents a key limitation as plasma levels alone may not fully capture local inflammatory activity. Supporting this, Shaheen et al. reported that YKL-40 expression in skin tissue samples was significantly higher in patients with moderate PV compared to those with mild disease. Moreover, in this study, both serum and tissue YKL-40 levels showed a strong correlation with PASI scores [12]. The study underscores the potential value of combining systemic and tissue-based assessments of YKL-40 in evaluating disease severity in PV.
An important addition to the existing observations is a study conducted by Imai et al., which included not only patients with PV but also patients with a more severe form of the disease – GPP. The analysis revealed that serum YKL-40 levels were significantly elevated in both patients with PV and GPP compared to the control group, with values being markedly higher in the GPP group. These results suggest that YKL-40 levels may increase in proportion to the clinical severity of psoriasis. Furthermore, significant correlations were observed between YKL-40 levels and markers of inflammation, such as WBC count, neutrophils, and CRP, in patients with GPP. The results reinforce the notion that neutrophils contribute to YKL-40 production during the course of the disease. Furthermore, treatment was associated with a significant reduction of YKL-40 levels, which coincided with clinical amelioration of cutaneous manifestations. However, the authors did not provide specific details regarding the therapeutic modalities employed [21].
In light of the aforementioned results, YKL-40 emerges as a promising biomarker, offering insights into the severity and progression of GPP. Nevertheless, the absence of data regarding the dynamics of this protein in relation to inflammatory markers in patients with PV remains a limitation, impeding a comprehensive assessment of its diagnostic and prognostic significance in this form of psoriasis. Similar observations were made in a group of patients with chronic generalized PV, in whom the mean serum concentration of YKL-40 was significantly higher compared to the control group. Moreover, this population showed a significant positive correlation between the level of YKL-40 and the severity of skin alterations assessed by the PASI scale [11]. An important methodological aspect of this study was the exclusion of patients with a diagnosis of PsA, which allows a clearer interpretation of the results.
Interesting observations also concern the relationship between YKL-40 levels and classic inflammation parameters in patients with psoriasis. Positive correlations have been demonstrated between YKL-40 concentration and CRP levels [16, 18], ESR [19], WBC count, and neutrophil count [18], particularly in patients with PV. However, these correlations were most pronounced in patients with more advanced, generalized forms of the disease, such as GPP [6]. In one study involving patients with PV, a significant correlation was found between YKL-40 and high-sensitivity CRP (hs-CRP), which was not observed with standard CRP [14]. These findings indicate that YKL-40 may serve as a marker of systemic inflammation, with its levels correlating with disease activity not only of the skin but also systemic involvement, particularly in advanced or generalized psoriasis.
Reliable data are provided by a study conducted by Jensen et al., which compared plasma YKL-40 levels in 48 patients with active, untreated PV, 42 patients with clinically active PsA, and a large control group of 3,130 healthy individuals. The results demonstrated that although the median YKL-40 concentration was higher in the PV group (45 μg/l) compared to the control group (40 μg/l), this difference did not reach statistical significance. In contrast, patients with PsA demonstrated a statistically significant increase in plasma YKL-40 levels prior to treatment (median 112 μg/l), compared to both the PV group and the healthy controls [14]. A similar pattern was observed in a study conducted by Imai et al., in which the mean serum YKL-40 level in patients with PsA was significantly higher (270 ng/ ml) compared to healthy volunteers (28.6 ng/ml) [15]. The significant increase in YKL-40 levels observed in patients with PsA is likely related to its secretion not only by inflammatory cells but also by human articular cartilage, chondrocytes, and synovial cells – sources that are not involved in PV. Notably, these studies, while demonstrating elevated YKL-40 levels in patients with PsA, did not reveal a statistically significant correlation between YKL-40 concentrations and PASI scores or the severity of joint disease [14, 15]. Only in the study conducted by Salomon et al. was a trend toward statistical significance observed between YKL-40 levels and PASI scores, although the result did not reach the threshold for significance (p = 0.071) [17]. These findings suggest that while YKL-40 is indeed associated with psoriatic disease, it cannot replace clinical severity indices such as PASI or Disease Activity Score in 28 joints – C-Reactive Protein (DAS28-CRP) at the time of diagnosis. It is plausible that YKL-40 reflects different aspects of the disease process beyond the visible severity of skin lesions, which warrants further investigation.
Interestingly, the study by Ahmed et al. reported a statistically significant increase in serum YKL-40 levels in both psoriasis patients without arthritis symptoms and PsA patients, compared to healthy controls. In this case, a statistically significant positive correlation was observed in both groups between serum YKL-40 levels and the Composite Psoriatic Disease Activity Index (CPDAI) score, which incorporates multiple domains of disease activity, including the skin component assessed by the PASI. A central finding of this study is derived from multiple regression analysis, which identified the PASI score as a significant predictor of YKL-40 levels. This observation is particularly noteworthy given the findings of previous studies, especially in the context of psoriasis without concomitant arthritis [22]. Perhaps an explanation for this discrepancy is that the patient groups analyzed also included patients with generalized psoriasis, which was not included or explicitly identified in the study methodology.
Importantly, elevated serum levels of YKL-40, in conjunction with its detection within the cytoplasm of neutrophils infiltrating the epidermis in skin samples from patients with GPP, imply that local production of this protein by keratinocytes or immune cells may play a pivotal role in perpetuating chronic inflammation [21]. This finding lends further credence to the hypothesis that YKL-40 functions not only as a peripheral marker of inflammation but also plays an active role in the pathogenesis of psoriatic lesions. However, despite these histological observations, topical therapies have not been shown to significantly reduce serum YKL-40 levels in patients with psoriasis [19]. This discrepancy may indicate that systemic inflammation, rather than epidermal turnover alone, is the primary driver of YKL-40 expression. Therefore, YKL-40 might be especially relevant in moderate-to-severe cases or those with systemic features.
Therapeutically, while no targeted treatments against YKL-40 currently exist, its modulation in response to phototherapy [18, 20], MTX [13], and biologic therapies [14, 15] supports its use as a pharmacodynamic marker. A study by El-Hamd et al. which evaluated the effect of narrow-band UVB (NB-UVB) phototherapy in patients with PV showed a significant decrease in serum YKL-40 levels, with a maximum reduction of 72.9% from baseline values after a 3-month treatment cycle [20]. In contrast, a study by Khashaba et al. conducted on a similar group of patients and using an identical duration of NB-UVB therapy showed a less pronounced, though still statistically significant, reduction in YKL-40 levels, by 28% [23]. This notable discrepancy in the results may be attributable to differences in UVB dosing protocols. In the El-Hamd et al. study, the dose was gradually increased by 10–20% per session, up to a maximum level of 5 J/cm², which may have contributed to a stronger therapeutic effect [20]. By comparison, Khashaba et al. did not report detailed information regarding the dosing regimen, representing a notable limitation in the interpretation of their results and in comparing treatment efficacy. Despite these differences, both studies demonstrated significant reductions in post-treatment PASIs, with a greater decrease in YKL-40 levels observed in patients with higher baseline PASI values [20, 23]. This finding may suggest a potential relationship between increased disease activity and the magnitude of marker response to NB-UVB treatment, indicating that patients with more severe baseline inflammation may exhibit a more pronounced decline in YKL-40 levels following therapy. Based on these results, YKL-40 could be considered a potential biomarker for evaluating NB-UVB treatment outcomes early and for ongoing monitoring of subclinical disease activity during maintenance therapy. A similar pattern has been observed in patients undergoing systemic treatment with MTX. MTX therapy resulted in a substantial decrease in YKL-40 concentrations in both the PV and PV + PsA groups, accompanied by clinical improvement as measured by PASI and DAPSA scores [13]. These findings suggest that YKL-40 may also reflect treatment response in joint-involved disease and support its potential utility as a biomarker.
In the context of biologic therapy for both PV and PsA, the YKL-40 marker shows potential value as an indicator of response to therapy, even though, as previously shown, its levels before treatment do not correlate unequivocally with clinical indices of disease activity, such as PASI or DAS28-CRP [14, 15]. In a study evaluating the effect of infliximab on YKL-40 levels in PsA patients, it was found that responders experienced a significant decrease in levels of this marker after just 6 weeks of therapy [15]. A similar phenomenon was observed with adalimumab therapy, although the dynamics of the marker response was different. In patients with PsA who responded to treatment, a significant decrease in plasma YKL-40 levels, to values typical of healthy subjects, occurred only after 48 weeks of therapy [14]. Although there was no correlation between YKL-40 levels and clinical severity as assessed by the PASI scale at baseline measurement, successful treatment with infliximab or adalimumab was associated with a concomitant decrease in both PASI values and YKL-40 levels. This suggests the potential usefulness of YKL-40 as a biomarker to monitor the effectiveness of biologic therapy in patients with psoriasis and PsA. It should be emphasized, however, that the timing of this response may depend on the specific biologic agent, the administered dose, and individual patient variability. These considerations further underscore the need for well-designed studies involving larger patient cohorts and stratified therapeutic approaches. In the original research conducted by Frątczak et al. [24], neutrophil gelatinase-associated lipocalin (NGAL) shows potential as a partial biomarker, particularly in detecting psoriasis-related itch and genital involvement, but it lacks the specificity and consistency needed for standalone use in monitoring disease severity or treatment progress. Therefore, pursuing the development of multi-biomarker panels is warranted as they hold promise for enhancing diagnostic accuracy and enabling more personalized therapeutic approaches in the management of psoriasis.
To summarize, while current findings position YKL-40 as a potential biomarker of disease activity and severity, its full significance in psoriasis pathophysiology and therapy remains to be established. Future research should prioritize longitudinal studies, explore mechanistic pathways and assess the biomarker potential of YKL-40 in conjunction with therapeutic outcomes. Additionally, efforts should be directed toward integrating YKL-40 into multi-biomarker panels that could improve diagnostic accuracy and guide personalized therapy in psoriasis. In line with this direction, our clinical team has initiated a research program aimed at validating the clinical utility of such multi-biomarker panels. This preliminary study represents the foundational step in assessing the efficacy and reliability of these composite biomarkers in monitoring therapeutic responses and stratifying treatment in psoriatic patients.
CONCLUSIONS
The investigation of YKL-40 as a potential inflammatory marker in psoriasis has yielded promising results. Specifically, elevated levels of YKL-40 have been observed in more severe, generalized forms of the disease, such as GPP and PsA. These findings suggest that YKL-40 production may be associated with the activation of various inflammatory cells, contributing to the inflammatory response in these cases. Although YKL-40 levels are typically elevated in patients, the correlation with skin lesion severity (PASI) remains ambiguous. This correlation is more pronounced in studies that incorporate both serum measurements and tissue expression. The observed decrease in YKL-40 after phototherapy, methotrexate treatment, and biologic therapy supports its usefulness as a marker of treatment response, although it does not replace standard clinical scales. Nevertheless, the necessity for additional well-designed studies encompassing larger patient groups and diverse psoriasis subtypes remains apparent. A comparison of YKL-40 with other inflammatory biomarkers could facilitate a more nuanced understanding of its diagnostic and prognostic value, as well as its potential role in future disease monitoring algorithms.