Introduction
Total knee arthroplasty (TKA) is an effective treatment for patients with persistently restricted joint function or pain, which is aimed to restore joint function, weight-bearing capacity and quality of life. Data have shown, for example in Germany, that the number of total knee arthroplasties in elderly patients is increasing, of which women are more frequently affected than men due to the higher prevalence of osteoarthritis and a significantly longer life expectancy for women [1]. Just like the survivorship of hip arthroplasty, in most cases the long-term survival rate of TKA was satisfactory, and sometimes re-revision surgery was needed for infection, loosening, polyethylene wear, or periprosthetic fractures [2, 3]. However, malignancies after TKA are rare.
Non-Hodgkin’s Lymphoma (NHL) is a malignancy of the lymphatic system of uncontrolled proliferation of B, T, or NK natural killer (NK)-cell lymphocytes, of which diffuse large B-cell lymphoma (DLBCL) is the most common subtype [4]. DLBCL, the most common lymphoid malignancy in adulthood, is a clinically and biologically heterogeneous disease and could be subdivided into germinal centre B-like (GCB) or activated B cell-like (ABC) subtypes according to the ‘cell of origin’ classification [5]. The pathogenesis of the disease is mainly genetic lesions induced dysfunction of proto-oncogenes and tumour suppressor genes as well as of other molecules of pathogenic significance, such as deregulation of BCL6 activity, constitutively activation of NF-κB, and chromosomal translocations of BCL2 and MYC [6]. Though age and genetic factors play a vital role in lymphomagenesis, other factors, especially environmental factors, are important contributing elements, of which chronic immune stimulation might render B-cell dysregulation and hyperactivity associated with impaired T-cell function [7].
Aim
Herein, we report the case of a patient with aggressive primary cutaneous diffuse large B-cell lymphoma within 1 year after TKA. The cutaneous mass develops around surgical sites, initially mimicking wound infection. However, common antibiotic treatment failed to improve the symptoms. After diagnosis of primary cutaneous diffuse large B-cell lymphoma (PCDLBCL) based on the pathological findings, the patient had a short-term response with rituximab, gemcitabine, and oxaliplatin (R-GemOx) incorporated immunochemotherapy. Then her condition turned worse and she died shortly. Giving its poor prognosis, the optimum therapeutic strategy for PCDLBCL was still unclear. Then, we reported an extensively systematic review of related articles of PCDLBCL. Our purpose was to 1) study the manifestations of PCDLBCL to understand the biological behaviour and aid early recognition of a flare up; 2) describe the effect and the outcome of treatment aiding to solve the problem of clinical decision-making of PCDLBCL.
Material and methods
We identified one patient with pathologically confirmed PCDLBCL at our institution. The detailed medical records were reported, including age at diagnosis, gender, clinical preoperative symptoms, duration, tumour location or related characteristics, treatment strategy, and follow-up outcome. The overall survival (OS) time was calculated using standard time to event analysis, which is defined as the time from confirmation of the diagnosis to the time of death or the end of follow-up. Complete remission (CR) was defined as regression of all measurable disease and clinical signs; partial remission (PR) was defined as a decrease of > 50% but < 100% in the sum of the products of the perpendicular diameters of all measurable tumours, and no response (NR) was defined as a decrease of < 50% or an increase in the sum of the products of the perpendicular diameters of all measurable tumours.
Sections of paraffin-embedded biopsy specimens were stained with H&E and immunohistochemical staining for the routine histologic diagnosis. The diagnosis was identified by the histological features and classified according to the World Health Organization (WHO) criteria.
Article selection and data extraction
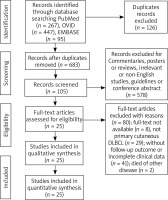
Two researchers independently searched PubMed, OVID, EMBASE for articles up to December 2020. Key words and corresponding Medical Subject Headings (MeSH) terms were used for searching databases. Search terms were utilized in various combinations: “Primary”, “Cutaneous”, “diffuse large B-cell lymphoma”, “Case Report”, combined with the Boolean operators (AND, OR, *). No limit was set. Inclusion criteria were articles published in the English language with PCDLBCL, diagnosed by pathology and reports with available clinical data. Exclusion criteria were: (1) patients with previous malignancy, (2) articles in non-English language, (3) unavailable or incomplete clinical data, (4) not primary cutaneous DLBCL or caused by infection or probable drugs, (5) death of other disease and (6) commentaries, posters or reviews, guidelines or conference abstract. This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Statistical analysis
Patients were divided into two groups according to the use of rituximab. Depict for survival was performed by comparing computer-generated curves estimated by the Kaplan-Meier method. Because of some unavailable or incomplete data, only univariate analysis was performed. The GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA) software was used for the statistical analyses. A p-value < 0.05 was considered significant.
Results
Our case’s clinical characteristic
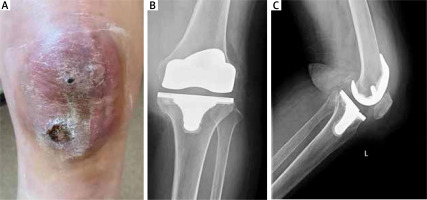
A 77-year-old female was referred to our hospital following a left knee TKA operation performed 1 year before. The procedure was routine, and postoperative recovery was uncomplicated until she found a gradually increased mass in the prepatellar area 3 months before without obvious symptoms, such as pain or burning sensations. The lesion was partially ulcerated. The prosthesis was in correct position without any sign of osteolysis (Figure 1). The recurrent ulcer around the surgery incision prompted the patient’s referral back to our unit. The patient had no systemic symptoms, and she denied any history of fevers, sweats, weight loss, or fatigue. She was also normotensive and had no family history of cancers or autoimmune conditions.
Figure 1
A – Cutaneous findings of the patient. A mass measuring 6.0 × 7.0 cm with partial ulceration on the left knee. B, C – The knee prosthesis was in the correct position without osteolysis
Most of the serum chemistry profile was within the normal values. A complete blood count (CBC) revealed no anaemia (haemoglobin level, 122 g/l), a normal platelet count (platelet count, 226 × 109/l), and a normal white blood cell count (WBC count, 3.89 × 109/l). However, biochemical analysis revealed an elevated erythrocyte sedimentation rate of 24 mm/h. Likewise, levels of C-reactive protein were also elevated at 11.06 mg/l. The levels of immunoglobulins, autoantibodies, and serum alexin were normal. Serum tests for Epstein-Barr virus and human cytomegalic virus DNA were negative. Furthermore, a radiograph of the knee and chest showed no abnormalities, and the arthroplasty was in the correct position. On physical examination, a firm, warm, painless, non-pulsating, dark red skin mass, about 6 × 7 cm, was evident (Figure 1 A). The patient achieved a range of active motion from 10 to 90° flexion without instability. Neither lymphadenopathy nor hepatosplenomegaly was detected. Neurological examination had revealed no abnormalities.
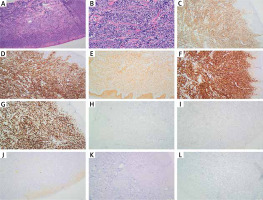
This skin mass was initially thought to be a prosthetic joint infection. Empiric antibiotic treatment was commenced, but it failed to elicit a response. At the same time, ulcer excreta cultures for bacteria (including extended incubation), mycobacteria were conducted and revealed negative. Thus, an infectious aetiology was considered less likely. As the nodules had persisted, a single punch biopsy of the lesion from the medial margin of the ulcer and the most indurated area was conducted. The pathological diagnosis of the mass was cutaneous diffuse large B-cell lymphoma, germinal centre B cell subtypes. The immunohistochemical stains of the mass were positive for CD10 (diffused), CD20, c-MYC (90%)-BCL-2 (90%) and Ki-67 in 80% of cells, but negative for CD2, CD3, CD4, CD5, CD7, CD8, CD21, CD30, CD56, MUM-1, EBER, S-100, HMB45, Melan-A, and BCL-6; genetic interrupt appeared in c-myc but not in BCL-2 and BCL-6. Diagnosis of primary cutaneous diffuse large B-cell lymphoma (PCLBCL), germinal centre B cell subtype was established. Typical details were shown in Figure 2. Examination of a bone marrow biopsy specimen and aspirate of iliac crest revealed maturing trilineage hematopoiesis, hypoplasia and a few lymphocytes were found and its atypia was not obvious, with no evidence of infection, lymphoma, or other tumour activity. Whole-body positron emission tomography/CT (PET/CT) with (18F) fluorodeoxyglucose (FDG) was performed, revealing lymphoma invaded medullary cavity of the femur and multiple hypermetabolic enlarged lymph nodes distributed along the left inguinal and iliac vessels and right popliteal fossa. Lungs and liver were not involved, thus stage IIE PCLBCL was diagnosed for this patient in accordance with Ann Arbor staging system.
Figure 2
Immunohistological findings. A, B – Needle core biopsy shows a diffuse lymphoid infiltrate; Haematoxylin and eosin, magnification 40×/200×, respectively. C – Diffuse immunopositivity for CD10. D – Positive staining for CD20. E – Positive staining for c-MYC. F – Positive staining for BCL-2. G – Positive staining for Ki-67. H – Negative staining for CD30. I – Negative staining for BCL-6. J – Negative staining for EBER. K – Negative staining for MPO. L – Negative staining for Mum-1
Systemic immunochemotherapy with rituximab, gemcitabine, and oxaliplatin (R-GemOx) was started. Although the skin lesions improved after chemotherapy, the disease had shown an aggressive course. The lesions decreased in the size into a tuberous appearance, with diameters ranging from 1 to 5 cm. After four cycles of chemotherapy, her PET/CT showed that the soft tissue mass at left knee joint mass reduced and the metabolic levels of FDG decreased, but its metabolic activity is still very high; previous lesions on the left side of inguinal and near iliac blood vessels lymph nodes disappeared. Her left superior genicular lymph node biopsy at that time showed CD10(+), CD19(+), CD20(+), CD79a(+), c-MYC(90%,+) BCL-2(90%,+) and Ki-67 in 90% of cells, but CD21(–), CD30(–), CD56, MUM-1(–), EBER(–), CyclinD1(–), and BCL-6(–). After five cycles of chemotherapy, her CT of the left knee and CT of the chest and CT of the abdomen and pelvis showed that a giant mass accompanied with enlarged lymph nodes can be found near the left medial femur and that her left side of lymph nodes of inguinal region swelled again. After nine cycles of chemotherapy, though reducing the dosage or switching to a different medication was conducted, she was not able to tolerate further chemotherapies due to her weak condition. The patient had problems with eating and drinking because of peritoneal metastasis. Then the patient declined additional treatment because her condition was progressive despite the treatment. Finally, she died from the diffuse large B-cell lymphoma after its onset within one year.
Data for literature cases
After exclusion on the basis of the title and abstract, and exclusion of commentaries, posters or reviews, guidelines or conference abstracts, and language other than English, 105 papers were assessed via full text for eligibility. Of these, 80 articles were excluded because of insufficient data, not primary cutaneous DLBCL, without follow-up outcome or died of other disease (Figure 3). The characteristics of the included 27 cases in 25 articles [8–32] with PCDLBCL are shown in Supplementary Table S1. Twenty-nine patients (13 male and 14 female) were identified up to December 2020 presenting with PCDLBCL in our review. The median age was 71 years, ranging from 41 to 92 years. Cutaneous manifestations were described in 24 patients: 79.1% patients (19 of 24 patients) presented infiltrative cutaneous lesions such as macules, papules or nodules, of which 16.7% of patients (4 of 24 patients) presented ulcerations or formation of vesicles; 20.8% of patients (5 of 24 patients) presented subcutaneous nodules; 4.2% of patients (1 of 24 patients) presented both cutaneous and subcutaneous lesions. The analysis of these data demonstrated that lower extremities are the main involved sites, followed by the trunk (including back and abdomen), superior extremities, breast, head (including face).
Initial treatment approaches for PCDLBCL in our data include surgical excision, radiotherapy (RT), and chemotherapy. The proportion of patients treated with chemotherapy was 77.8% (21 of 27 patients), R-CHOP or CHOP-like regimens was the most frequent initial treatment approach chosen (16 of 21 patients); 37.3% patients (10 of 27 patients) underwent local radiotherapy, while 14.8% patients (4 of 27 patients) performed surgery. The median follow-up was 18.5 months (range: 5–67 months).
Pooled analysis
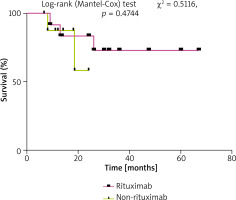
Because of limited data, the survival of patients who received radiotherapy or surgery was not included in our analysis. The survival time of patients who received systemic rituximab was better than in those who did not (Figure 4). This difference did not reach significance, but it trended toward it. In patients who received systemic rituximab chemotherapy, 10 of 12 (83.3%) patients had CR, 2 (16.7%) had PR, and 7 of 12 patients (58.3%) had recurrence/metastasis/progression. Of them, 4 patients with CR did not report any recurrence/metastasis/progression during the follow-up, and median time to the first recurrence/metastasis/progression for the rest of patients with CR was 14.25 months (range: 4–19.5 months). The chemotherapy, radiation, surgery or maintenance on rituximab was combined after the recurrence/metastasis/progression, 5 patients had a second remission and remained alive in the last follow-up, while one patient was dead after CNS metastasis.
Discussion
To our knowledge, this is the first report of aggressive primary cutaneous diffuse large B-cell lymphoma, initially manifested as a probably infected mass around the surgery region of TKA, which might be associated with chronic inflammation. In our case, the patient had been treated by empiric antibiotic for an initial period of a few days. At the same time, we consulted our dermatology team and then a punch biopsy of the mass was conducted, which allowed us to diagnose the presence of a cancer. Then, the patient was informed of these findings and was referred to the haematology team for staging and subsequent treatment.
Large B-cell lymphoma after knee arthroplasty is very rare, with only 8 other reported cases in the literature (Supplementary Table S2) [33–40]. All of them were detected in the tissue around prosthesis, such as soft tissue, bone, synovium, and joint aspirate, whose histology was consistent with DLBCL or LBCL. Of which, diffuse large B-cell lymphoma (DLBCL) germinal-centre subtype was the most subtype reported after knee arthroplasty and R-CHOP combined with radiotherapy was the most frequent therapy. Based on the above literature, the clinical presentation was varied, including fever of unknown origin, swelling, pain, lytic bone lesions and prosthetic loosening, even altered mental status, which provided a diagnostic challenge, with symptoms and signs mimicking other conditions. Thus, chronic inflammatory reaction around the knee prosthesis should be noted, which might not only be associated with prosthesis loosening but also carcinogenesis, of which large B-cell lymphoma was more common in elderly patients with arthroplasty.
Primary cutaneous lymphomas (PCLs), non-Hodgkin lymphomas that present in the skin, are the second most common group of extranodal non-Hodgkin lymphomas after gastrointestinal lymphomas [41]. Furthermore, according to the 2020 updated WHO-EORTC classification for primary cutaneous B-cell lymphomas (PCBCLs), five types are recognised: primary cutaneous marginal zone lymphoma (PCMZL), primary cutaneous follicle-centre cell lymphoma (PCFCL), and diffuse large B-cell lymphoma, leg type (PCDLBCL, LT), intravascular large B-cell lymphoma, and EBV-positive mucocutaneous ulcer (EBV-MCU) (Supplementary Table S3) [42]. As for the classification of CBCLs with histologic features of a DLBCL, 2 types were recognised according to 2005 WHO-EORTC classification: PCDLBCL, LT, and PCFCL with a diffuse growth pattern (Supplementary Table S4); and in rare cases that cannot be classified as either PCDLBCL, LT, or PCFCL, a diagnosis of diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS) should be made [43]. According to the literature, PCDLBCL-LT classically expresses BCL2, IRF4/MUM-1, and FOXP1 and also commonly expresses BCL6, but typically lacks CD10, whose fluorescent hybridization may show both c-myc, bcl-6, and bcl-2 mutations [44, 45]. Furthermore, the immunophenotype of PCDLBCL-LT should be non-germinal centre B-cell derived [43]. In our case, though the lesion involved the skin of the lower leg, whose origin was germinal centre B cell, considering that the expression of CD10 was positive, the expression of BCL6 was negative, and genetic interruption was only found in c-myc, we tend to classify them as DLBCL, NOS.
Rituximab-based chemoimmunotherapy is the preferred first-line treatment of diffuse large B cell lymphoma (DLBCL), and accumulating evidence demonstrated that dose attenuation does not compromise survival in the very elderly. Thus, R-CHOP (rituximab, cyclophosphamide, vincristine, prednisolone), especially R-CHOP-21 (every 21 days) remains a standard of care for most of these diseases, which can provide equivalent efficacy and a superior toxicity profile [46]. With the development of molecular biology, things were new. It has been demonstrated that co-expression of MYC and BCL2 protein has an inferior outcome for patients treated with R-CHOP, which is associated with an aggressive clinical course [47, 48]. Thus, it is imperative to find some effective method for those who failed to respond to R-CHOP mode. Currently, some researchers found that R-GemOx can also function as the first-line treatment in patients with DLBCL, which has shown high efficacy with a low toxicity profile in elderly patients with relapsed and refractory DLBCL [49–51]. In our case, her immunohistochemical stains of c-MYC and BCL-2 were positive, so we directly chose R-GemOx, which had previously shown high efficacy and good tolerability in the elderly patient. However, the patient only had a response in the initial four cycles of treatment, which only maintained for less than 4 months. In the further treatment, we tried to reduce the dosage or switch to a different medication due to her worse condition. Following treatment, her overall condition got better, in the ninth chemotherapy cycle, we continued R-GemOx regimens. However, after that her condition turned even worse, she developed pulmonary infection, abdominal infection, heart failure, atrial fibrillation, and electrolyte disturbance during the hospital stay. The patient did not tolerate further treatment and died shortly.
Chronic inflammation is a precursor of most tumours, and Epstein Barr virus infection is often implicated in primary cutaneous diffuse large B-cell lymphoma, which is concerned with chronic inflammatory stimulation [52, 53]. Pro-inflammatory mediator not only participates in acute infectious diseases but is also involved in the evolution of sterile inflammation [54]. For example, pro-inflammatory transcription factors NF-κB can regulate more than 500 cancer-related genes [55]. Similarly, chronic inflammation after arthroplasty can also have an increased risk of malignancy. And according to the reported cases, most of the malignancies were soft tissue around the prosthesis. It has been proved that accumulation of wear debris can induce peri-implant osteolysis due to host response of chronic inflammation, which is associated with activation of NF-κB signal [56]; in that way, the excessive existence of wear debris might constitutively activate NF-κB signalling pathway, which might be associated with lymphomagenesis. For the first time, our case indicates that primary cutaneous B-cell lymphoma may arise in association with arthroplasty. Nonetheless, the reason for the development of lymphoma is most likely to be multifactorial, of which metal, surgical trauma, chronic inflammation, or host factors are all a potential element.
Conclusions
We illustrate a very rare case of a 77-year-old woman diagnosed with PCDLBCL after TKA.
The main cutaneous manifestations of PCDLBCL include infiltrative cutaneous lesions such as macules, papules or nodules, some of them presented ulcerations or formation of vesicles, subcutaneous nodules or both. And the treatment options include excision, radiotherapy, chemotherapy, and even “watchful waiting”. Though the difference was not significant, patients who received systemic chemotherapy with rituximab tend to have a better overall survival (OS) time than those who did not. However, due to the limitations of the case study and small sample size, the most useful therapeutic method is still hard to figure out.