Introduction
Psoriasis is a common, chronic, inflammatory skin disease. Increased keratinocyte proliferation plays a role in its pathogenesis. The clinical picture and location of skin lesions are different in individual age groups [1]. One-third of psoriasis cases begin in childhood [2–4]. Genetic, immunological and environmental factors play a role in the development of this disease [5]. The onset of the disease in childhood may herald its severe course. Moreover the early onset of psoriasis and the associated deterioration in quality of life and negative self-perception may have implications for children’s psychological development, increasing the risk of anxiety and depressive disorders in adulthood [6, 7]. In recent years, an increased incidence in moderate and severe psoriasis in children has been observed. Currently, psoriasis is perceived as a systemic inflammatory process. Clinical observations and scientific research showed that adult psoriatic patients were predisposed to development of cardiovascular diseases, metabolic syndrome and its signs such as abdominal obesity, hyperlipidaemia, hypertension, insulin resistance, abnormal glucose tolerance, as well as to development of non-alcoholic fatty liver disease, type 2 diabetes, inflammatory bowel diseases, autoimmune diseases, psoriatic arthritis, psychological disorders [8, 9]. An increased risk of those comorbidities can also occur in the paediatric population but studies confirming the relationship are still being conducted [10–12]. The prevalence of metabolic syndrome in patients with psoriasis ranges from 20% to 50%. The risk of developing metabolic syndrome is at least twice as high in patients with psoriasis. In one analysis of the global prevalence of metabolic syndrome among patients with psoriasis, the prevalence in adults was 32%, while in children and adolescents it was 9% [10, 13]. Psoriasis and cardiovascular diseases demonstrate common etiopathogenetic features and may be mediated by the same proinflammatory cytokines (e.g. TNF-a, IL-1, IL-6, IL-8, IL-12, IFN-g, IL-17, IL-22, IL-23). The production of proinflammatory cytokines is also increased in obese individuals, which may contribute to exacerbation of psoriasis. A lot of authors confirmed that the occurrence of metabolic syndrome and obesity contributed to the development of psoriasis and its more severe course, while a greater severity of psoriatic lesions increased the cardiometabolic risk [14, 15]. Early identification of risk factors of concomitant diseases in psoriatic children is likely to prevent the disease from developing in adulthood. Psoriasis may affect children’s mental health and quality of life.
Aim
The analysis of cases of children diagnosed with psoriasis depending on sex, age and medical history, determination of the incidence of concomitant diseases and their risk factors by assessing abnormalities in laboratory findings in the study group, comparison of psoriatic children with normal weight, overweight and obesity in relation to the severity of psoriatic lesions, analysis of the impact of disease duration on the assessment in the PASI, BSA (Body Surface Area), CDLQI (Children’s Dermatology Life Quality Index) and FDLQI (Family Dermatology Life Quality Index) scales, assessment of quality of life of the patients and their families, providing interdisciplinary medical care to psoriatic patients with concomitant metabolic disorders and other diseases, educating patients and their families about psoriasis, collecting data on the methods of psoriasis treatment in the paediatric population.
Material and methods
The study was conducted with the approval of the Bioethics Committee at the Medical University of Lodz, according to the Declaration of Helsinki principles, with the written, informed consent of the patients and their legal guardians.
The study included 99 psoriatic children, aged 2–17 years (61 girls and 38 boys). They were hospitalized in 3 paediatric dermatology centres from March 2020 to March 2022. The severity of the disease was assessed during the first visit using the PASI and the affected skin area index (BSA). The CDLQI and FDLQI were used to assess the quality of life of the patients and their families. Body weight and height were measured to determine BMI. The result calculated with a standard formula was referenced to age- and sex-specific norms included in the percentile grids. They were considered normal (< 85th and > 5th percentile), overweight (≥ 85th and < 95th percentile) or obese (≥ 95th percentile). In order to assess obesity and fat distribution in the patient’s body, the WHR index (waist-hip ratio) was determined. The WHR is the quotient of measurements of waist circumference to hip circumference. Waist circumference was measured midway between the highest point of the iliac crest and the lowest point of the lower costal margin at the end of normal exhalation, using a non-stretchable anthropometric tape, in the standing, upright, and relaxed positions with upper limbs aligned along the sides of the torso and feet positioned close together. The analysis was carried out on the basis of sex- and age-specific percentile grids [16, 17]. Medical data were collected from the patients (Tables 1 and 2). Systolic and diastolic blood pressure was measured. The measurement was taken in each child after a 5-minute rest in the sitting position on the right shoulder. An automatic measuring device was used with a cuff matching the size of the child’s arm, placed halfway between the acromion and olecranon, covering the entire circumference of the arm and at least 2/3 of its length. The result of the blood pressure measurement was compared to the relevant reference tables, depending on sex, age and height. Laboratory blood tests were performed after an overnight fast to assess the risk of diseases concomitant with psoriasis. The influence of disease duration on the assessment in the PASI, BSA, CDLQI and FDLQI scales was analysed.
Table 1
Medical history data
Table 2
Factors preceding the occurrence of the first-ever psoriatic lesions and exacerbating the course of the disease
Statistical analysis
Univariate linear regression was used in the analysis. Results are presented as b coefficient with corresponding confidence intervals (95% CI) and p-values. Information was collected on how patients had been treated until they were enrolled in the study. During the first examination, the patient was educated about the disease. For this purpose, a psoriasis knowledge guide and a psoriasis knowledge test consisting of 30 questions were developed for patients and their caregivers.
Results
All 99 patients had psoriasis vulgaris. The PASI score ≥ 10, indicating moderate and severe psoriasis, was obtained in 56% of the participants, while the PASI score < 10, indicating mild psoriasis, was obtained in 44% of the participants. As regards normal-weight patients, PASI ≥ 10 was obtained in 46% of the subjects, and with regard to overweight and obese patients, PASI ≥ 10 was obtained in 100% of the subjects. The Body Surface Area index of psoriatic lesions ≥ 10 was obtained in 58% of all the participants, while the index value < 10 was obtained in 42% of the participants. As regards normal-weight patients, BSA ≥ 10 was obtained in 46% of the subjects, and with regard to overweight and obese patients, BSA ≥ 10 was obtained in 100% of the subjects. The quality of life of paediatric psoriasis patients and their families was significantly reduced, 57% of all patients demonstrated CDLQI ≥ 10, and 62% of all patient caregivers had FDLQI ≥ 10. Among patients with CDLQI ≥ 10, 14% had mild psoriasis (as measured by PASI). Among caregivers with FDLQI ≥ 10, 21% had mild psoriasis (as measured by PASI).
46% of all patients had one exacerbation of the disease per year, 34% of all patients had two exacerbations of the disease per year, 13% had 3 exacerbations of the disease per year, and 7% had no remission periods. Pruritus was observed in 44% of the patients and arthralgia in 11% of the patients. Location of the first skin lesions in life was head in 34% of the patients, upper limbs in 23%, trunk in 18%, lower limbs in 9%, reproductive organs in 2%, nails in 2%, and generalized skin lesions in 1% of the patients.
BMI above the reference range for age and sex was found in 19% of the patients. Overweight was confirmed in 1% of the people and obesity in 18%. WHR above normal range for age and sex was noted in 18% of the patients. Abnormal blood pressure was observed in 1%. Comorbidities other than obesity were observed in 28% of patients: hypertension in 1% of the subjects, insulin resistance in 1%, psoriatic arthritis in 1%, subclinical hypothyroidism in 2%, symptomatic hypothyroidism in 1%, Hashimoto’s disease in 3%, celiac disease in 2%, bronchial asthma in 4%, uveitis in 1%, psychological disorders in 5%, non-alcoholic fatty liver disease in 3%, acquired vitiligo in 2%, nocturnal enuresis in 2%, nephrotic syndrome in 1%, severe dental caries in 4%, cerebral palsy in 1%, short stature treated with growth hormone in 1%, vesicoureteral reflux in 1%, and Down syndrome in 1% of subjects.
Abnormalities in blood laboratory results were observed in the examined patients (Table 3). Lipid disorders were observed both in patients with normal body weight and in obese and overweight ones. With regard to patients with abnormal body weight, lipid disorders were observed in 42% of the subjects. In normal-weight patients with psoriasis, lipid disorders occurred in 16%. We also confirmed abnormalities concerning thyroid hormonal balance, levels of liver enzyme, uric acid and glucose. Besides, the presence of microcytic anemia was another abnormal manifestation. In addition, we noted increased antistreptolysin O (ASO) titre level, inflammatory parameters and antibodies characteristic of celiac disease. Patients with abnormalities detected in laboratory tests but without previous disease diagnoses were referred to appropriate specialists for further diagnosis. Treatment administered in children between the diagnosis and inclusion in the study, according to the history collected from the caregivers was topical medications (cignolin ointment, topical glucocorticosteroids, tacrolimus) in 100% of the patients, acitretin in 9%, methotrexate in 4%, cyclosporine in 3%, UVB-NB phototherapy in 2%, and etanercept in 3%.
Table 3
Abnormalities in laboratory tests
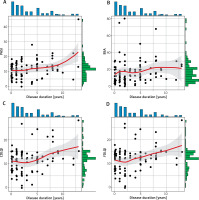
In the tests of knowledge about psoriasis, a result of below 60% was obtained by 28% of patients. The duration of the disease has a statistically significant impact on the assessment in the PASI, CDLQI and FDLQI scales, indicating an increase in the values of these scales with the increase in the duration of the disease (Table 4, Figure 1).
Table 4
One-factor analysis of the influence of the disease duration on the assessment in the PASI, BSA, CDLQI and FDLQI scales
Discussion
Psoriasis vulgaris is the most common form of psoriasis in children. The disease affects girls rather than boys. A significant number of patients with moderate and severe disease is observed in the paediatric population. Our study revealed that in children with psoriasis concomitant with obesity, the severity of psoriatic lesions as measured by PASI and the area of skin involved (BSA) were higher compared to children with normal body weight, which confirmed that a higher BMI was correlated with the severity of psoriasis. In our study the disease onset occurred before the age of 10 in the majority of the patients. Upper respiratory tract infections and psychological stress were the most common factors preceding the occurrence of the first skin lesions in life and causing exacerbations in the course of the disease. Pruritus in the area of skin lesions was a frequently reported symptom in the paediatric population. The first skin lesions were most often located within the scalp. A positive family history of psoriasis was common in the study group of children. Most patients experienced one exacerbation per year. All the examined children received topical treatment. Some patients with moderate and severe disease received systemic treatment and UVB-NB phototherapy. In children, cardiovascular diseases are rare, while the observed occurrence of lipid disorders and an increase in uric acid levels may promote their development in adulthood [18]. Confirmed seropositivity regarding thyroid autoimmune diseases and celiac disease without clinical symptoms may reflect subclinical autoimmunity or an increased susceptibility to autoimmune diseases. Our study showed that lipid disorders occurred both in obese and overweight patients as well as in those with normal body weight. Abnormalities in the concentration of thyroid hormones and liver enzymes were observed, without accompanying clinical symptoms, which may indicate an increased tendency to develop thyroid and liver diseases. An abnormal fasting glucose level indicates increased likelihood of development of diabetes in psoriatic individuals. Elevated levels of antistreptolysin O (ASO) titre and concentrations of C-reactive protein (CRP) confirm that the presence of active inflammation and infection may exacerbate the disease.
Psoriasis begins in childhood in almost one-third of patients. Early identification of risk factors of concomitant diseases in psoriasis children may reduce the likelihood of their development in adulthood. Issues indicative of an increased cardiometabolic risk in our patients include hypertension, insulin resistance and abnormal fasting glucose. Psoriasis is an independent risk factor of comorbidities, and obesity associated with psoriatic lesions may contribute to increase the cardiometabolic risk. Non-alcoholic fatty liver disease, bronchial asthma, uveitis, microcytic anemia and psychological disorders may be concomitant with psoriasis. Concomitant autoimmune diseases also occurred in the study group and they included Hashimoto’s disease, celiac disease and acquired vitiligo. However, this relationship requires further observations. In our centre, we conducted a retrospective study among children with psoriasis hospitalized in the Dermatology Department in the period from 2018 to 2020. We also observed the presence of concomitant diseases such as obesity, hypertension, Hashimoto’s disease, coeliac disease, non-alcoholic fatty liver disease and the presence of abnormalities in blood tests that may indicate an increased cardiometabolic risk [19]. According to the opinion of the Polish Society for the Treatment of Obesity, in Poland excessive weight and obesity has been increasingly observed in children and adolescents in recent years [20]. Our study revealed a significant association between psoriasis and obesity, which turned out to be the most common comorbidity. Paller et al. conducted a multicentre study and observed that children with psoriasis were more likely to be overweight or obese and, therefore, they were at increased risk of cardiometabolic diseases, which required early monitoring of health and lifestyle modifications [21]. Moreover, a multicentre study conducted in France showed that obesity was more common in children with psoriasis than in the control group [22]. A lot of factors may contribute to coexistence of obesity and psoriasis. They include eating habits, lifestyle as well as the presence of chronic inflammation characteristics of the diseases. The adipose tissue is considered an endocrine organ, contributing to increased production of proinflammatory mediators [23–25]. Obesity may be concomitant with psoriasis but it may also precede the occurrence of the first-ever psoriatic lesions and contribute to their development. Obesity concomitant with psoriasis was found to exacerbate psoriatic lesions, contribute to a more severe course of the disease and poorer response to treatment [26–28]. In our study, moderate to severe severity of psoriatic lesions was observed in all obese patients, which may confirm the relationship between obesity and greater severity of psoriasis [29].
Psoriasis may be concomitant with metabolic syndrome. This association was confirmed in the adult population. All components of metabolic syndrome increase the risk of cardiovascular disease. The occurrence of metabolic syndrome was defined according to the 2007 International Diabetes Foundation criteria. Metabolic syndrome includes hypertension, obesity, carbohydrate metabolism disorders and dyslipidemia [30, 31]. Hypertriglyceridemia, increased total cholesterol, LDL cholesterol and decreased HDL cholesterol, as well as disturbed carbohydrate metabolism were observed in the paediatric patients included in our study. Abnormal lipid levels were observed in obese patients and in those with normal body weight. No patient met criteria for the full-blown metabolic syndrome, while individual patients had particular features of metabolic syndrome, which may indicate an increased cardiometabolic risk. Similar results were obtained by Koebnick et al., who conducted a study on a large population of children suffering from psoriasis [32]. A study conducted in India showed an occurrence of full-blown metabolic syndrome in psoriatic children [33]. An increased incidence of metabolic syndrome, lipid disorders, overweight and obesity were also demonstrated in a study conducted by Pietrzak et al. on a group of 965 children [9].
Patients with psoriasis are at increased risk of hypertension [34]. Our study included 1 patient with hypertension concomitant with psoriasis. An Italian study including children with mild to severe psoriasis without associated metabolic disorders revealed elevated blood pressure values in the study group compared to the control group. Patients with psoriasis should be routinely examined for hypertension [35, 36].
Psoriasis may be concomitant with autoimmune diseases. We observed celiac disease, acquired vitiligo and Hashimoto’s disease in our study group [37–39]. The co-occurrence of psoriasis and autoimmune diseases results from common pathogenetic mechanisms involving the inflammatory aetiology and the presence of common gene loci.
Non-alcoholic fatty liver disease (NAFLD) is characterized by excessive accumulation of lipids in hepatocytes. Many studies confirmed that this disease, due to common inflammatory aetiology, may be concomitant with psoriasis [40]. In our study, NAFLD was observed in 3 patients. A study including 52 children with psoriasis also showed a higher incidence of NAFLD, particularly in children with moderate to severe psoriasis, concomitant obesity or reduced HDL cholesterol [41].
Psoriasis may be associated with an increased risk of bronchial asthma, which may result from common inflammatory aetiology of both diseases. However, this relationship requires further observations. In our study, bronchial asthma was observed in 4 patients. A Danish study showed a significantly increased risk of bronchial asthma in patients with psoriasis [42].
Numerous reports found in professional literature indicated a greater risk of mental disorders in psoriasis, especially manifested by anxiety and depressive disorders, and significant mood worsening. The occurrence of psoriasis has a negative impact on the quality of life of patients and their families [43, 44].
Education is necessary to increase the awareness of patients and their families of the pathophysiology of psoriasis, factors aggravating the course of the disease, lack of infectious nature of the disease, lack of restrictions in everyday life activities and the need to modify lifestyle due to the impact of environmental factors on the development of the disease as well as the possibility of development of pruritus (scratching the eruptions intensifies the symptoms – the Koebner phenomenon) and the ability to properly apply general and topical treatment. Broadened knowledge will contribute to therapeutic success and more effective adherence of patients to medical recommendations.
Conclusions
Children and adolescents with psoriasis are often obese and with abnormal lipid profile, which may contribute to the development of cardiovascular diseases. Higher BMI is correlated with the greater severity of psoriasis. The disease has a negative impact on the quality of life of patients and their families. The quality of life index is poorer regardless of the severity of psoriasis, which confirmed that even mild skin lesions might deteriorate the patients’ frame of mind. Longer disease duration can make psoriatic lesions more severe and worsen patients’ quality of life. Patients with paediatric psoriasis should be routinely screened for risk factors of comorbidities, which may significantly reduce comorbidity-related mortality in adulthood.
It is important to provide such patients with lifestyle counselling. People suffering from psoriasis should maintain normal body weight, use a healthy diet and be physically active regularly. Appropriate treatment of psoriatic lesions and knowledge of exacerbating factors may improve the quality of life of patients.