Acute febrile neutrophilic dermatosis (Sweet’s syndrome – SS) is an uncommon inflammatory disorder characterized by suddenly appearing fever, tender skin lesions, neutrophilia and dense infiltrate of predominantly mature neutrophils in skin biopsy [1]. Typically purple-red papules, nodules or plaques are described whereas pustular lesions are not a commonly-reported finding [2]. Here we report the case of a 53-year-old woman who presented with SS with predominant pustular lesions and a literature review on selected neutrophilic dermatoses which may present pustular eruptions.
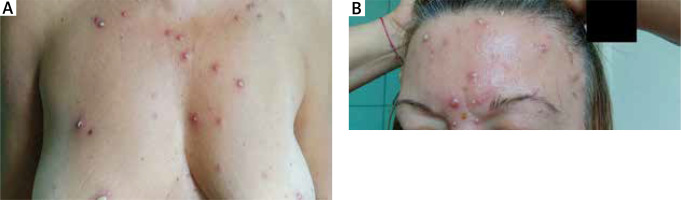
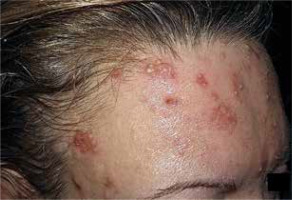
A 53-year-old woman was admitted to the Dermatology Department due to a tender, nonpruritic pustular rash that developed 3 days before, accompanied by fever up to 40°C, muscle pain and malaise. Clinical examination showed pustules varying in size 0.5–2 cm and erythematosus papules on her face, trunk and upper extremities (Figure 1) with no mucous membrane involvement. She was otherwise healthy and did not take any medications, aside from levonorgestrel-releasing intrauterine device for 1 year. Laboratory tests showed raised total white cell count 21.30 (4–10) × 109/l with neutrophilia 86% (40–70%), C-reactive protein 130.93 (0–5) mg/l, erythrocyte sedimentation rate (ESR) 70 (2–15) mm/h. Aerobic and mycological culture from the pus was negative, anaerobic showed Cutibacterium acnes growth. The sinus X-ray revealed a haze of the right maxillary and frontal sinus. Upon admission, the patient was empirically started on intravenous cefuroxime. She remained febrile and on the 8th hospital day, a new lesion erupted on the forehead giving the impression of herpes zoster infection (Figure 2). Therefore, acyclovir was added but the lesions kept worsening. Blood and urine culture were negative. Further investigations showed negative results of IgM antibodies for Varicella zoster virus, Herpes simplex virus, Coxsackie A7 and B1 as well as HIV virus, HCV antibodies, HBs antigen, antinuclear antibodies, antineutrophil cytoplasmic antibodies and rheumatoid factor. To rule out bullous diseases a direct immunofluorescence study was made, but we did not detect any deposits.
A biopsy specimen revealed acute inflammatory infiltrate in the upper dermis with neutrophils and papillary dermis oedema with no evidence of vasculitis.
Histological features and laboratory findings were consistent with SS diagnosis so methylprednisolone of 40 mg daily was initiated. After 2 days of treatment, marked improvement was observed with complete resolution of the skin lesions within 2 weeks. The intrauterine device was removed and the screening for haematological changes was made, with the negative outcome. She was followed for 1 year and had no relapse during this period.
Pustular lesions were found in 20% of patients in Nofal’s report and described in only few more reports in the literature which makes it a very rare variant of SS [3]. The lesions usually appear as erythematous-based pustules up to 2 cm in diameter, or tiny pustules overlying an indurated plaque, showing disseminated, grouped or confluent distribution. Eruptions are usually accompanied by typical plaques and nodules, rarely by additional vesicles and haemorrhagic blisters [4–8]. In differential diagnosis, bullous diseases, vasculitis, viral and bacterial infections are taken into account, however additional findings ultimately exclude these entities. The most commonly associated disorders with pustular variant are haematological neoplasms, solid tumours and infections [4, 6–10]. After initiation of systemic corticosteroids and/or dapsone therapy, rapid improvement is observed, similarly to those with idiopathic SS.
This rare variant of SS should be distinguished from cases where pustular changes are predominantly localized on the dorsal hands, known as neutrophilic dermatosis of the dorsal hands (NDDH) or acral neutrophilic dermatosis [11, 12]. NDDH is considered as a distinct entity rather than a localized form of SS due to some separate clinical, histological and laboratory features [12]. In our patient hands were not involved, she presented with severe systemic symptoms and the vasculitis was not seen in biopsy so NDDH was excluded.
Another neutrophilic dermatosis that may present with pustular eruptions is bowel-associated dermatosis-arthritis syndrome (BADAS), which may correspond to previously described “pustular eruption of ulcerative colitis” or atypical SS [13, 14]. Eruptions are usually located over the trunk and extremities and are associated with arthralgia, fever and neutrophilic infiltrate in the superficial dermis in histopathology. Unlike patients diagnosed with BADAS, our patient did not reveal any gastrointestinal disorder or undergo any bariatric procedure.
Behçet disease was the least likely considering the classical triad (recurrent painful oral and genital ulcers, and uveitis, multisystem involvement and chronic course) which the patient did not present [1]. Papulopustular lesions, however, are accepted as one of the minor diagnostic criteria, and for that reason this rare neutrophilic dermatosis should be kept in mind in the differentials.
The traditional classification of SS differentiates between idiopathic (or classic), malignancy-associated and drug-induced SS. Idiopathic form usually affects middle-aged women with preceding infection, presence of pregnancy or inflammatory disorders. Several drugs were reported to cause SS with contraceptives as an infrequent triggering agent [2].
Recently, Nofal et al. has proposed new diagnostic criteria for establishing the diagnosis of SS. Previous major and minor criteria were replaced by constant features (abrupt onset of painful erythematous plaques or nodules and dense dermal neutrophilic infiltrate), which are sufficient to make a diagnosis and variable features whose absence does not exclude the diagnosis [3]. Pustular lesions were classified as atypical skin lesions among variable features. Moreover, they proposed these diagnostic criteria for classical as well as drug-induced SS.
Our patients fulfilled two constant and six variable features (fever, atypical skin lesions, absence of leukocytoclastic vasculitis, elevated ESR and C-reactive protein levels, leukocytosis), however, in the clinical picture, tender pustules were dominant, unlike the nodules or plaques. We did not find any underlying disease in addition to signs of sinusitis which may be referred to as the trigger factor. Levonorgestrel-induced SS was reported in the literature, however, temporal relationship between drug ingestion and clinical presentations of SS in our case (1 year) is questionable [15].
SS, though rare, should be considered as a differential diagnosis of pustular lesions especially when a patient is unresponsive to experiential antimicrobial and antiviral therapy. This may quicken accurate diagnosis and detect the potential underlying disease.