Generalized pustular psoriasis (GPP) is an intractable autoinflammatory skin disease characterized by diffused erythematous rashes and recurrent flares of pustular [1]. Effisayil™ 1 trial showed the IL-36 antibody spesolimab could bring fast clearance of pustules and lesions and excellent patient safety profile, which made it the first treatment option in the GPP flare approved by the United States (US) [2, 3]. However, the efficacy and safety of spesolimab in Chinese population has not been clearly reported. We herein reported a Chinese case with a GPP flare who is heavily treated with other biologics but with poor response, and was successfully treated with spesolimab.
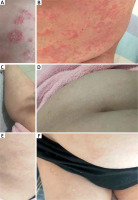
A 34-year-old female patient visited our department due to a 2-year history of GPP with a poor treatment effect. She had a history of recurrent plaque-type psoriasis for 20 years which had been treated with topical glucocorticoids. She had no family history of psoriasis and other skin diseases. Two years ago, the patient experienced an extensive edematous erythema covered with dense pustules, as well as pyrexia (maximum body temperature of 39.0°C) after delivery. She presented to the local hospital and was diagnosed as GPP. Then oral Chinese medicine was prescribed considering her lactation period but with poor efficacy. When breast-feeding ended, the patient’s treatment was adjusted to oral acitretin (20 mg/day) and traditional Chinese medicine. The rash was slightly better than before, but was accompanied by recurrence of pustules with varying degrees. The patient’s latest relapse had occurred after an upper respiratory infection leading to massive exacerbation of GPP with high fever. Two months prior to visiting our department, she had been treated unsuccessfully with two distinct biologic classes including anti-IL-12/23 (ustekinumab 45 mg) once, as well as anti-tumor necrosis factor (TNF)-α (adalimumab 80 mg) twice with an interval of 2 weeks in another hospital which induced only a temporary improvement. Her condition had been stabilized in approximately a week, however, new pustules appeared later. At the time of presentation, the patient presented with generalized erythema and scattered pustules on the trunk without pyrexia. Laboratory tests indicated that the blood routine, C-reactive protein, hepatic and renal functions, infection tests like HIV, syphilis, hepatitis B and C, T-SPOT were all negative. She had a Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) total score and GPPGA pustulation subscore of 2 and Dermatology Life Quality Index (DLQI) score of 18 (Figures 1 A, B). Given her poor response to various treatments previously, she participated in an expanded access program in China with a single intravenous dose of spesolimab 900 mg. She had completed the standard laboratory test and signed the informed consent. During follow-up, in addition to spesolimab, she was allowed to take acitretin (20 mg/day) orally if necessary. The patient exhibited improvement of erythema and the pustules disappeared especially fast within 2 days. Complete remission had been achieved within 1 week (GPPGA pustulation/total score: 0 and DLQI: 2) (Figures 1 C, D) and no relapse of pustules or side effects were observed during the following 5 months (GPPGA pustulation/total score: 0/1 and DLQI: 3) of follow-up (Figures 1 E, F). The patient provided written informed consent for publication of this case report.
Figure 1
A, B – Cutaneous manifestations before spesolimab therapy, C, D – a complete remission of pustules and erythema after 1 week of spesolimab treatment, E, F – an almost complete clearance of skin lesions 5 months after one dose of spesolimab 900 mg
GPP flares can be life-threatening without appropriate treatment, however therapy is very challenging. The episodic feature and mutable severity of GPP flares posed challenges in designing high-quality clinical trials leading to the absence of globally accepted guidelines for GPP patients. Currently, by targeting different pathogenic cytokines biologics may improve the clinical outcome of GPP which have been approved in a few countries [4–7]. However, off-label use of these biologics is mainly based on limited evidence from small studies, and still some patients with poor response.
In our case, the patient’s initial response to acitretin was slow and it could not completely resolve her symptoms. In the meantime, she refused to use cyclosporine or methotrexate due to the potential cumulative toxicities and limited efficacy. Afterwards, treatment with 45 mg ustekinumab was initiated in another hospital, her fever resolved within 48 h and pustules disappeared within 1 week, however it failed to prevent relapse of new flares. Then the treatment was switched to adalimumab 80 mg every 2 weeks, only with initial improvement but with no long-term efficacy in preventing recurrence of pustules. Considering the intermittent remission and spontaneously episodic pustular flare features of GPP, consequently, there is an urgent need for a drug that can show a long-term efficacy in this patient.
In recent years, the Effisayil™ 1 trial demonstrated the efficacy and safety of spesolimab on GPP flares [2], this study excluded patients who had used restricted biologics (2 months prior to the visit). Spesolimab is a humanized ani-IL-36R monoclonal antibody by inhibiting the key IL-36 pathogenetic pathway and downstream inflammatory activation in GPP [8]. However, the efficacy and safety of spesolimab in Chinese population have not been reported. We shared 1 case in whom she just failed from biologics, however was successfully treated with spesolimab. Compared with previous treatments, she demonstrated rapid clinical response and sustained remission with spesolimab monotherapy. Pustules were completely cleared within 48 h after treatment. A GPPGA pustulation score and total score of 0 (clear skin) were achieved in this patient by week 1 (Figures 1 C, D) with a significant decline of DLQI score to 2 points. Until 3 months after treatment, she had a mild relapse of psoriasis rash due to respiratory infection, but with no new pustules. Therefore, the patient was given acitretin 20 mg/day orally and the topical glucocorticoids until rashes had subsided. At the time of writing, 5 months have passed since spesolimab treatment, and she has been in an almost complete remission of lesions with no adverse events (Figures 1 E, F).
To our knowledge, this case is the first report demonstrating successful and safe use of spesolimab in a refractory Chinese GPP patient who showed resistance to ustekinumab and adalimumab treatment. Simultaneously, it is encouraging that this case also enriches the real-world patient data of the GPP patient treated with spesolimab beyond the exclusion criteria of Effisayil™ 1 study. And the long-term follow-up will be further conducted to monitor possible relapses as well as any potential adverse effects. In summary, we suggest spesolimab may trigger a more rapid and sustained therapeutic response, making it a better choice for patients with GPP flares.








