Introduction
Allergic diseases are an important challenge for medicine and public health as their prevalence continues to increase with the civilization progress. According to one of the opinions, food allergies present a particular problem as their incidence has nearly doubled over the last decade. The condition affects mainly children (4.7–8% of the general population, particularly in infants and children below the age of 3), with the prevalence among adults ranging between 1% and 2% [1–3]. The most frequently encountered food allergens include cow’s milk proteins. According to questionnaire surveys, hypersensitivity is declared in 1–17.5% of pre-school children, 1–13.5% of older children, and around 4% of adults [4]. In addition to the conventional differential diagnosis of food allergens, the oral provocation test known as the Goldman (open challenge) test or its blinded placebo-controlled modification is considered the gold standard in the diagnostics of food allergies [5]. The assessments are made on the basis of reported gastrointestinal symptoms as manifested by the subject during the course of the test. In contrast to the other type of provocation test, i.e. nasal provocation test, the oral challenge test is rather a subjective assessment made by the study subject [6]. In the literature, increasing attention is being paid to the potential for extending the applicability of nasal allergen provocation tests (NAPTs) in differential diagnostics of food allergies. Nasal cavity is a well-vascularized area which contains a large number of mast cells; importantly, it is an easily-accessible organ for subjective and objective evaluation of both early and late allergic reactions. Unexpected results were obtained in a murine model study carried out by French and American researchers to investigate the differences in responses to the inhaled peanut allergen within the nasal cavity and the gastrointestinal tract. The allergen (administered orally and intranasally) triggered significant changes at the cellular level causing respiratory hyperreactivity [7]. Dose standardization and determination of threshold values for positive NAPT results appear to be the most important challenges for the standardization of NAPT as a diagnostic tool in food allergies.
Aim
The objective of the study was to assess the reactivity of nasal mucosa to local application of a cow’s milk protein allergen in the form of powdered milk as widely used in the food industry. The choice of the study area was dictated by the demand for a specific alternative to oral provocation tests which, due to its difficulty, lack of objectivity and limited patient access, present with certain disadvantages of a purely technical as well as informative nature.
Material and methods
A total of 31 subjects (mean age: 26.47 (21–39), mean height 170.2 (154–183), mean weight 64.7 (46–86), 22 women and 9 men, Table 1) with no sensitivity to cow’s milk protein as determined from medical history and differential diagnostic examinations were included in the study. Inclusion criteria were: negative clinical history and negative diagnostic screening tests, including allergy skin tests; the exclusion criteria consisted of the obligatory criteria for the qualification of patients to nasal allergen provocation tests, including acute bacterial infection within the nasal cavity and sinuses, severe comorbidities (malignancies, autoimmune diseases), systemic immunotherapy, or pregnancy. Other relative exclusion criteria included nasal deformation, choanal atresia, septal perforation, severe septal deviation, nasal polyps, atrophic rhinitis, adenoid hypertrophy, acute allergic reaction from another organ, vaccinations within the week preceding the NAPT, acute viral or bacterial infection (4 weeks), nose and sinus surgery (6–8 weeks), alcohol consumption and smoking within 24–48 h before the NAPT [6].
Table 1
Study group characteristics
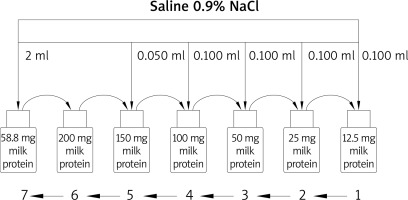
The study method consisted in the nasal allergen provocation test carried out in accordance with the applicable standard procedure [6]. The nasal allergen provocation test was performed at three time points: at the baseline, following application of the control solution, and following local application of the allergen at incremental doses. The reactivity of nasal mucosa was assessed by means of two techniques including the subjective visual analog scale (VAS, 10-cm scale for assessing the degree of nasal discomfort) and the objective acoustic rhinometry method. The nasal patency in the course of the nasal allergen provocation test was assessed using acoustic rhinometry on the basis of the analysis of the minimum cross-sectional areas as measured within the nasal cavity at the following levels: MCA1 – cross-section corresponding to the nasal isthmus (0–2.5 cm on the Y-axis of the rhinometric curve) and MCA2 – head of the inferior nasal concha (2.5–4.5 cm on the Y-axis of the rhinometric curve). Mucosal reactivity was determined from the difference in mean MCA1 and MCA2 values (separately for the left and right side of the nasal cavity as well as for the entire nasal cavity). In addition, the tryptase levels were measured in nasal lavage fluid at three time points: within 48 h before the test (to minimize the risk of mucosal hyperreactivity), after administration of the control solution, and after the local allergen application. Nasal lavage was collected using the Greiff method [4] and centrifuged in a laboratory centrifuge (15 min at 1000 rpm). In the resulting supernatant, the tryptase concentration was determined using the immunofluorescence method on the ImmunoCAP platform (ThermoFisher Scientific; previously known as Phadia). In the so-called incremental challenge test, the provocation material consisted of powdered cow’s milk (100 g containing: 1.25 g of fat, 51 g of carbohydrates, and 34 g of protein) with the following doses (prepared under laboratory conditions) being administered from a calibrated nasal atomizer [1] according to the study protocol: 12.5 μg, 25 μg, 50 μg, 100 μg, 150 μg, 200 μg, and 58.8 g (Figure 1).
All subjects signed the Bioethics Committee’s approval (KB 65/2021); the research was carried out within the framework of the Warsaw Medical University grant no. PW/Z/2/2/20(1).
Statistical analysis
For the purpose of the statistical analysis of the results, the Student’s paired t-test was used, and the differences in the measured parameters were verified by means of the Bonferroni correction applied at the three study time points: prior to the nasal allergen provocation test, following the administration of the control solution, and following the local allergen application. The statistical significance threshold was defined as p < 0.05.
Results
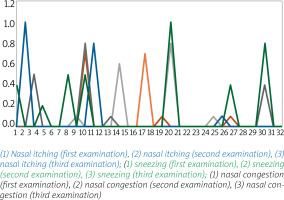
Minor changes were observed within the range of subjective complaints as measured by the VAS scale (Figure 2) at various stages of the study, namely nasal itching (first test: 0.013 ±0.07, control solution 0.023 ±0.127, local allergen application 0.053 ±0.179), watery discharge (no complaints in the first test; second test 0.063 ±0.229, control solution 0.126 ±0.127, local allergen application 0.263 ±0.179). The differences were not statistically significant and consisted mainly of minor nasal itching.
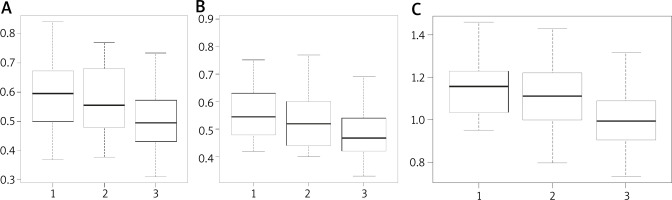
In line with the minor changes within the discomforts as assessed using the VAS scale, changes in MCA1 (Figure 3) were observed, particularly within the left nasal cavity (first test: 0.4958 ±0.085, control solution 0.4885 ±0.084, local application of the allergen 0.417 ±0.100). The differences were statistically significant (Student’s t-test, significant differences between stages 2 and 3: p = 0.004226 × 3 = 0.012678 with Bonferroni correction for 3 test stages) and between stages 1 and 3 (p = 0.001874 × 3 = 0.005622 with Bonferroni correction for 3 test stages). No significant differences were observed in relation to the left side of the nasal cavity and the entire nasal cavity (both sides).
Figure 3
Cross-section of the nasal isthmus (MCA1): A – MCA1 for the right side of nasal cavity, B – MCA1 for the left side of nasal cavity, C – MCA1 for the total nasal cavity (left and right side combined)
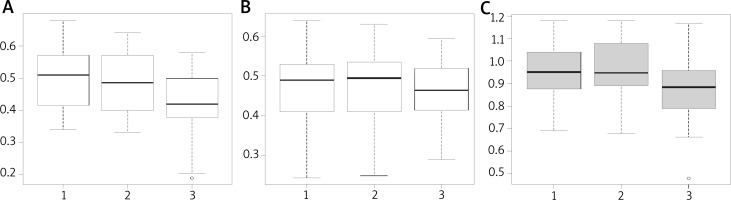
The reactivity within the head of the inferior nasal concha was significantly more varied, with differences being observed within the left as well as the right side of the nasal cavity (Figure 4), thus ruling out the nasal cycle phenomenon. On average, changes observed over time already after the first applied dose of 12.5 μg of protein were as follows: 1.160 ±0.134, control solution 1.112 ±0.161, local allergen application 1.005 ±0.157. A statistically significant decrease in nasal patency was observed between the tests using the control solution and the allergen, respectively (paired Student’s t-test, p = 0.01149 × 3 = 0.03447 with Bonferroni correction for 3 comparisons), and between the baseline and the allergen application, respectively (p = 0.0001296 × 3 = 0.0003888 with Bonferroni correction for 3 comparisons). The increased reactivity of nasal cavity mucosa was observed on the left side as compared to the right side of the nasal cavity (left: 0.561 ±0.5618, control solution 0.571 ±0.109, local allergen application 0.489 ±0.083 at p = 0.005276 × 3 = 0.015828; right: 0.598 ±0.108, control solution 0.541 ±0.104, local allergen application 0.516 ±0.11 at p = 0.005275 × 3 = 0.015825). Slight differences of no statistical significance were observed in the levels of tryptase in the nasal lavage fluid (first test: 68.8 ±6.54, control solution 63.2 ±7.01, local allergen application 73.4 ±5.6). Due to the significant decrease in nasal patency as measured by means of the minimum cross-sectional area in acoustic rhinometry studies, further tests with increasing doses of the allergen were abandoned.
Discussion
The study was inspired by the potential for the use of NAPTs in the diagnostics of cow’s milk protein allergies. Due to the rich symptomatology and the variety of causative factors, the diagnostics of food allergies is a difficult and tedious, frequently multi-step process [8]. The standards for the management of suspected food allergies include detailed clinical history, physical examination, skin tests (prick tests, native tests, atopic patch tests), laboratory investigations (specific component-resolved diagnostics (CRD), basophil activation test (BAT)) and elimination diets. In the light of the current medical knowledge, challenge tests play the key role in the diagnostics of food allergies. The double-blinded placebo-controlled food challenge (DBPCFC) test remains the most important examination considered to be the gold standard in the diagnostics of food allergies [9, 10]. The main indication for the challenge test is to confirm the causal relationship between the consumption of a particular food and the hypersensitivity reaction. The challenge test is used to recreate and mimic the natural systemic response to the administered allergen. The positive result of the challenge test identifies the perpetrator and elimination diet usually follows. It is important to note that any diagnostic challenge test is associated with a potential risk of arduous or dangerous symptoms. Taking into account the risk of anaphylaxis, the test should be carried out in a hospital setting and the patient should be followed up for at least 24 h after the test. In addition, the performance of placebo-controlled challenge tests requires specific skills and adequate diagnostic facilities with regard to the masking of study foods and placebo, the monitoring of the test, and objectification of its results [9, 10]. All the above factors encourage the search for an alternative diagnostic tool which would reproduce the natural response of the body to the tested food on the one hand while being safer, more accessible for patients, and feasible in an outpatient setting on the other. Kvenshagen and Jacobsen highlighted the need to look for novel modalities in the diagnostics of food allergies due to the increased prevalence of these allergies and to the potential risks, high costs and time demands of oral challenge tests. Based on a review of the available publications, the authors proposed using allergen challenges, i.e. nasal, conjunctival, and labial allergen provocation tests, as well as endoscopically monitored food challenge tests. The methods were considered promising due to the availability of mucous membranes in the aforementioned anatomical locations and the possibility of low doses of the allergen being used [11]. The nasal allergen provocation test consists in the allergen being administered in the form of a solution onto the nasal mucosa. As a result of application, the test follows the natural history including itching at 1 min, sneezing at 2–3 min, and increased serous discharge followed by mucosal swelling at about 10 min. The nasal allergen provocation tests are widely used in the diagnostics of allergic rhinitis, local allergic rhinitis (LAR), and are considered to be conclusive in the event of discrepancies between the clinical interview and the skin prick and sIgE tests, particularly when qualifying the patient for specific immunotherapy and as part of research studies. Nasal provocation tests are relatively safe diagnostic examinations. The immediate reaction symptoms usually resolve within several minutes. Symptoms of increased intensity or symptoms from organs other than the nose require adequate treatment, but severe complications are rather rare. For this reason, nasal allergen provocation tests may be carried out in an outpatient setting [11]. An additional argument for the use of nasal allergen provocation tests in the diagnostics of food allergies consists in clinical symptoms within the upper airways being frequently observed in oral food challenge trials [10]. In addition, food allergies may develop through inhalation exposure. Smeekens et al. used a murine model to demonstrate sensitization to inhaled peanut allergens pointing to the significant role of house dust components as the adjuvant in allergy induction [12]. A case of inhalation allergy to milk thistle in a patient who had never eaten the plant in the past was also presented by our study team [13]. In recent years, the issue of the environmental factors promoting the development of food allergies such as environmental pollution, exposure to inhaled allergens, particularly pollen, and the supply of vitamin D3 [14, 15] is also raised.
However, little data are available in the literature on the use of nasal allergen provocation tests in the diagnostics of food allergies. In 1985, Amlot et al. presented the results of a study involving the use of nasal, labial, and gastric provocation in 39 patients with oral allergy to milk and hen’s eggs as diagnosed from the clinical history positive skin prick test results. The results of the nasal provocation tests were assessed on the basis of PNIF measurements and the number of sneezes. No oral food challenge test was performed. On the basis of the obtained results, the nasal allergen provocation test was considered to be the most sensitive modality [15]. Seppey et al. and Clark et al. presented the results of the studies involving nasal allergen provocation tests being performed with hen’s egg and peanut allergens. Facial thermography was used by the authors to assess the test results; the examination was considered to be rapid, safe, and objective [16–18]. Gelis et al. presented the results of an interesting study assessing the usefulness of the nasal allergen provocation test in the diagnostics of shellfish allergies and in the differentiation of patients with allergy and non-allergic hypersensitivity as an alternative to the oral food challenge test. The study included a total of 45 people with a shellfish allergy confirmed by means of the skin prick test, nasal allergen provocation test, history of anaphylaxis or intolerance to shellfish in medical history. The control group consisted of 10 healthy individuals. Boiled shrimp lyophilisate was used for the nasal test and the results were assessed on the basis of acoustic rhinometry and visual analog scale. The results confirmed the usefulness of the nasal allergen provocation test in the diagnostics of shellfish food allergies [19].
In our study, we decided to examine the reactivity of nasal mucosa to the cow’s milk allergens in a nasal allergen provocation test in healthy volunteers using the non-purified product as commonly used in the food industry. Positive, non-immune responses were observed in the subjects. Probably, the responses consisted of mediator-type reactions involving the responses from the sympathetic system, the parasympathetic system, and the receptors present in the nasal mucosa [20, 21]. This reaction was probably due to the effects of substances contained in drinking milk such as fats and carbohydrates. In its character, the course of the nasal allergen provocation test was similar to that of the non-specific provocation test using cold air, histamine, or methacholine [22]. Therefore, appropriate preparation of the allergen material for nasal allergen provocation tests seems to be of key importance, with the allergen being adequately purified and standardized to eliminate the potential false positive results.
Conclusions
When used in the nasal allergen provocation test, a product of widespread use in the food industry (powdered cow’s milk) is associated with the risk of non-specific false positive responses thus supporting the need for further research into the standardization of this test method. In this respect, appropriate preparation, purification (establishment of a methodology to prepare the allergen by laboratory methods) and accurate dosage of food allergens for the purpose of nasal allergen provocation tests appear to be the key issues.