Introduction
Chronic inflammatory skin diseases such as psoriasis (PS) and hidradenitis suppurativa (HS) are among the most common skin diseases globally and are classified as systemic diseases. A high inflammatory burden is frequently observed in patients and can lead to comorbidities or increase their severity if the disease is not adequately controlled. Systemic therapies such as biologics, which have shown their effectiveness in decreasing the inflammatory load, are therefore crucial treatment options [1].
According to the guidelines of the Drug Commission of the German Medical Association (AkdÄ), biologics contain “active substances that are either biological substances, are of biological origin or are produced from biological source material. In most cases, these are proteins that are produced in a complex biotechnological process in living, genetically modified microorganisms or cells”. Biologics are anti-inflammatory molecules that specifically intervene in disease processes as monoclonal antibodies. Currently approved substances in Germany include tumor necrosis factor (TNF)-α blockers such as etanercept, adalimumab or infliximab and many other active substances (interleukin IL-12/23, IL-23 and IL-17 antagonists). Since the initial approval of etanercept in 2004, there has been a continuous advancement in TNF-α blocker development. This has resulted in a sharp increase in the number of applications and indications, as well as a significant rise in treatment costs. At the same time, there is constant pressure on healthcare systems to decrease costs. Consequently, pharmaceutical companies are eager to develop biosimilars, which are medical replicas of a corresponding biologic drug, as a means of providing continued access to these medications after the patent rights of the original substance have expired [1]. Biosimilar approvals have currently been granted by the European Medicines Agency (EMA) for adalimumab, etanercept and infliximab [2]. Although biosimilars do not expand the range of therapies, through their noticeable cost reduction, they bring significant economic benefits. As a result, a selection of such biosimilars is now extensively used in the field of dermatology [1].
The European Union legal framework for biosimilar approval, introduced in 2004, must be complied with as a prerequisite for biosimilar approval. Every medicine or drug produced using biotechnology must be approved by the EMA before being commercially available. When the manufacturer applies to the EMA for approval, the necessary data is “evaluated by the Scientific Committees on Human Medicines and Safety […] as well as by the experts on biological medicinal products […] and by specialists on biosimilars […]” [3]. The EMA then provides its findings to the European Commission, which is responsible for the Europe-wide market authorization. The equivalence of clinical efficacy and safety to the original product are essential for approval [1]. This process must be shown in clinical trials only for one disease. The targeted biosimilar is then confirmed for authorization for all other diseases, for which the original biologic is approved [3].
Various smaller studies have already dealt with this comparability between biologics and biosimilars [4, 5], but real-world data regarding patient benefit, efficacy and particularly the subjective tolerability on the patient’s side is sparse.
Dermatological diseases, such as PS or HS, are particularly suitable for comparing the equivalence of biosimilars and biologics as their therapeutically effect is far more pronounced than in other indications [1].
Aim
To establish a rationale for the responsible approach to the prescription of biosimilars, this observational study aims to investigate real-world data focused on patient-specific factors such as the spectrum of side effects and individual tolerability as well as treatment response.
In doing so, retrospective data on outpatients treated in the outpatient clinic for inflammatory skin diseases at the Department of Dermatology and Department of Rheumatology of the University Medical Center Mainz was collected. This data was analysed to assess the burden of disease, the side effect rate and symptoms occurring during and following the transition from a biologic to a biosimilar and, if applicable, during switching back to the originator drug. The focus of the study was to examine whether biosimilars (adalimumab, etanercept) can be prescribed as a replacement and demonstrate comparable treatment response to that of the original biological agents (TNF-α blockers) in the treatment of inflammatory skin diseases. At the time of this study, available adalimumab biosimilars were Hulio®, Imraldi®, Amgevita®, and Hyrimoz®, while Benepali®, Erelzi®, and Nepexto® were available as potential etanercept substitutes.
Material and methods
All patients treated at the outpatient clinic of the Department of Dermatology and Department of Rheumatology of the University Medical Center Mainz with PS (with/or without psoriatic arthritis) or HS from January 2019 to June 2021 were retrospectively assessed, after obtaining approval from the ethics committee. Patients were included if they had been treated with a biologic (adalimumab, etanercept) for at least 3 months HS. Follow-up assessment took place between 3 to 9 months after switching from the biologic to the biosimilar and, if necessary, after switching back to the original substance. Dermatological co-medication, such as concomitant methotrexate therapy, was maintained. No patients in our cohorts developed new comorbidities such as psoriatic arthritis or inflammatory bowel disease during the observation period. Statistical analysis was performed using paired t-test. Differences were considered statistically significant if p < 0.05.
Results
A total of 259 outpatients were retrospectively examined. 94% of them suffered from PS (72% men, 28% women, aged 12–88 years) and 6% from HS (31% men, 69% women, aged 25–55 years). The majority of these patients (198/259, 76.4%) had previously received Humira® (adalimumab) and 23.6% (61/259) had been treated with Enbrel® (etanercept). Analysis of all 259 patients, sorted according to diagnosis, who underwent therapy with biologics, showed that 74.9% of patients with PS (182/243) received Humira® and 25.1% (61/243) Enbrel®. All HS patients had been treated with Humira®. A total of n = 206 (79.5%) of all 259 patients were switched to a biosimilar. Fifty-three patients were not switched to a biosimilar due to other severe inflammatory comorbidities (such as Crohn’s disease or ulcerative colitis), as well as therapy discontinuation. In addition, there was a group of patients who were directly treated with a biosimilar (Supplementary Figure S1).
Switch to a biosimilar
Of the analysed cohort, 206 patients (79.5%; 193 PS and 13 HS) under therapy with biologics were switched to a biosimilar, 8.2% (13/159) of all adalimumab patients were switched to Amgevita®, 58.5% (93/159) to Hulio®, 0.6% (1/159) to Hyrimoz® and 32.7% (52/159) to Imraldi®. The etanercept patients were switched to Benepali® (68%), to Erelzi® (27.7%) and to Nepexto® (4.3%). Based on the diagnoses, half of the PS patients (48.2%; 93/193) were switched to Hulio®. 21.8% of patients (42/193) to Imraldi®, 16.6% (32/193) to Benepali®, 6.7% (13/193) to Erelzi®, 5.2% (10/193) to Amgevita®, 1.0% (2/193) to Nepexto® and 0.5% (1/193) to Hyrimoz®. Among HS patients, 76.9% (10/13) were switched to Imraldi® and 23.1% (3/13) to Amgevita®.
Of the patients that switched medication, 94.2% (194/206; 181 PS and 13 HS) showed up for follow-up assessment in our outpatient clinic. Psoriasis Area and Severity Index (PASI) was available in 132 and Dermatology Life Quality Index (DLQI) in 73 of these patients. 86/194 (44.3%) patients presented again within the first 3 months after switching, 85/194 (43.8%) patients between 3 and 6 months, 20/194 (10.3%) patients between 6 and 9 months and 3/194 (1.54%) patients after more than 9 months (not shown). Mean follow-up time was 16 weeks.
64.9% (126/194) of these patients had no complaints after switching to a biosimilar (Figure 1 A).
Figure 1
A – Complaints on the first follow-up appointment. 35.1% (68/194) of the patients presented with symptoms and side effects at the first follow-up appointment. B – Effect on the skin and joints after switching to a biosimilar during the first follow-up appointment. 82% of the patients reported an improvement or stable disease progression of skin and 79.4% of joints
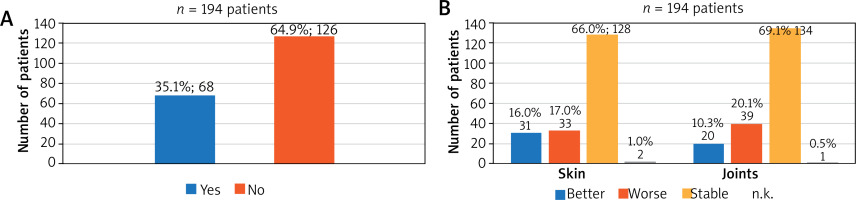
35.1% (68/194) of the patients who presented again at the first follow-up appointment reported complaints (symptoms or side effects), such as deterioration in efficacy, headaches, gastrointestinal problems, fatigue or pain at the injection site.
Reasons for switching back to the biologic at the first follow-up appointment
More than half of the group (51.5%, 35/68) reported complaints in the form of a deterioration in efficacy – compared to the biologic treatment – since the start of biosimilar therapy. As side effects, 8.8% (6/68) reported headaches and 5.9% (4/68) reported gastrointestinal problems and fatigue. A further 8.8% (6/68) of patients described/complained of pain at the injection site. 45.6% (31/68) of the group reported a wide variety of complaints, which were grouped under “Other”. These included abscesses, dysphagia, flushes, hyperhidrosis, itching, circulatory problems, panic attacks and pain (not shown).
Skin symptoms of patients switched to the biosimilar
16.0% (31/194) of the patients reported an improvement in skin condition and 66.0% (128/194) reported stable findings. Thus, change in therapy had a positive or non-deteriorating effect on 82.0% (159/194) of the patients (Figure 1 B). There were no significant differences in PASI (available in 132 patients, p = 0.23) and DLQI (available in 73 patients, p = 0.31) between the therapy switch time and the follow-up.
When analysing the data separately for the diagnoses PS and HS, it became apparent that 69.1% (125/181) of the PS patients reported a stable skin condition and 15.5% (28/181) reported an improvement. In contrast, 23.1% (3/13) of the HS patients reported an improvement in their skin and 23.1% (3/13) reported a stable condition. While 53.8% (7/13) of the HS patients reported a deterioration, only 14.4% (26/181) of the PS patients complained (not shown).
Joint symptoms regardless of preexisting conditions in patients switched to biosimilars
Analysis of the joint symptoms showed that 10.3% (20/194) of patients felt an improvement, 20.1% (39/194) described worse joint symptoms and 69.1% (134/194) of patients reported a stable condition (Figure 1 B). 52.7% (128/243) of these patients were previously diagnosed with psoriatic arthritis.
Diagnosis-dependent analysis (PS vs. HS) showed that 68.0% (123/181) of the PS patients reported a stable condition and 11.0% (20/181) reported an improvement. As many as 84.6% (11/13) of the HS patients reported a stable joint condition; however, no improvements (0.0%) were reported. 15.4% (2/13) of patients in the HS group experienced deterioration, compared with 20.4% (37/181) in the PS group (not shown).
Therapy with biosimilars and switching back to the biologic
In 78.9% (153/194) of the overall switched patient group, the biosimilar therapy was continued until the end of the observation period (of 21 to 27 months). 21.1% of the overall switched patients (41/194, 35 PS and 6 HS; 34 in the adalimumab group and 7 in the etanercept group) were switched back to the original substance (25/34 (73.5%) to Humira® and 7/7 (100%) to Enbrel®) or another medication (9/34 (26.5%)) until the end of the observation period. 35.1% (68/194) of the patients complained after switching to the biosimilar. In 41.2% (28/68, 24 PS and 4 HS) of these patients, the complaints at the first follow-up appointment were so significant that they were switched back to the biologic or another medication depending on the physicians and patients’ decision. Another 13 patients were re-treated with the biologic in the observation period, adding to a total of 41 patients who were switched back. 25/41 (61.0%) patients were switched back to the biologic adalimumab (including 4 HS patients at the first follow-up appointment) and 7/41 (17.0%) were switched back to etanercept. 9/41 patients (22.0%) were prescribed another therapy with a different active ingredient, e.g. antibiotics in 2 HS patients or guselkumab, ixekizumab, etc. in psoriatic patients (Figure 2 A). Five patients (20.0%, 5/25) that were initially switched to adalimumab were transitioned to an alternative therapeutic option during the follow-up visit within the observation period.
Figure 2
Switching back from a biosimilar to the biologic treatment (A). The total number of patients switching back to the original treatment – reasons (B). Of the total of 41 patients who switched back, 25/41 patients (61.0%) returned to the biologic treatment with adalimumab, Humira®, and 7/41 patients (17.0%) to etanercept, Enbrel®. 9/41 patients (22.0%) were prescribed a different medication, e.g. antibiotics, guselkumab, ixekizumab, etc. (A). Deterioration in efficacy (68.3%), progression of arthritis (68.3%) and skin symptoms (56.1%) were the main reasons given for switching back, whereas fatigue, headaches, gastrointestinal problems or pain at the injection site were rarely mentioned (B)
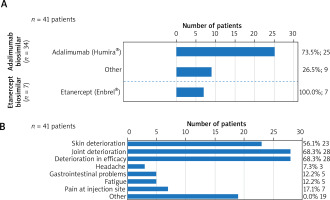
Reasons for switching back to the biologic at the end of the study
Overall patients’ and investigators’ reasons to return to the previous treatment included uncontrollable disease activity with regards to loss of efficacy (68.3%, 28/41), progression of joint pain (68.3%, 28/41) and increased skin symptoms (56.1%, 23/41). Interestingly, fatigue, headaches, gastrointestinal problems, or pain at the injection site were rarely reported (Figure 2 B).
Discussion
In our observational cohort, 206 patients were switched from the biologic to the biosimilar, of which 159 (77%) were previously treated with Humira® and 47 (23%) with Enbrel®.
It should be noted that only a few studies have been conducted in patients with PS and HS, so that an expansion of the amount of available data on this topic is encouraged. There are currently no published studies in the literature that investigated a switch from Humira® to several different adalimumab biosimilars. Therefore, no direct comparison can be made here. However, there are a few studies that deal with the switch from adalimumab to Amgevita® (PS and HS patients) [5–7], to Imraldi® (rheumatoid arthritis (RA) patients) [8, 9], to Hyrimoz® (PS and RA patients) [10] and to Hulio® (RA) [11]. All these studies – except HS patients – showed that adalimumab biosimilars have equivalent efficacy, immunogenicity and tolerability compared to their originator [2]. The randomized double-blind clinical trial by Papp et al. [5] and the results of Gooderham et al. [12] also show that the efficacy of Amgevita® is comparable to that of adalimumab in PS treatment. There were no differences in the incidence of adverse events or anti-drug antibodies (ADA) between the groups examined. Gooderham et al. showed that the numbers of ADA were comparable in all 3 groups (treatment with adalimumab, treatment with Amgevita®, treatment with adalimumab and switch to Amgevita®) [2, 12]. Kirsten et al. reported on 94 HS patients, who were switched from biologic to biosimilar treatment and found that about 33% experienced a loss of response or adverse events, suggesting that switching between treatments may not always yield the same clinical outcomes [7].
Again, currently no published studies are available, which investigate a switch from Enbrel® to several different biosimilars within the same study. Consequently, no direct data comparison is possible. However, there are a few studies in which the switch from etanercept to Benepali® took place, but only in patients with RA [13]. Nevertheless, there are some studies that describe the switch from Enbrel® to Benepali®. For example, the 2017 survey by Emery et al. shows no differences in terms of efficacy and response, with only 5.0% of patients in the switched group and 5.6% of patients who received a biosimilar directly discontinuing treatment prematurely. 1.7% of the switched group and 3.2% of the biosimilar group discontinued treatment due to adverse events [14, 15]. The data from Hendricks et al. from 2017 also shows that after the switch, 11.0% of patients discontinued treatment after 4 months [15, 16]. Discontinuation rates were clearly lower than in our study. In addition, the EGALITY study by Griffiths et al. investigated the switch from Enbrel® to Erelzi® in PS patients [4]. This study also showed comparable results in terms of efficacy, immunogenicity and tolerability between the biologic and the biosimilar, which was proven by an assessment of disease activity [2]. Therefore, further studies in larger cohorts are required.
According to the study by Rabbitts et al. from 2017, 84.0% of patients continued to be treated with the biosimilar Benepali® 1 year after the switch. There were only 5 treatment discontinuations due to loss of efficacy [15, 17]. Similarly, the survey by Sigurdardottir et al. [18] shows that 86.0% of the evaluated patients who switched from Enbrel® to Benepali® were still being treated with it after 10 months. Only 9 of the 147 patients were switched back to Enbrel® [15, 18]. In the study by Holroyd et al. [19], as many as 91.0% of patients continued etanercept after switching to the biosimilar with no change in efficacy [15, 19]. The results were comparable with our study and can be reflected in the figures regarding the PS patients.
While a total of 14.4% of the switched patient group was switched back at the first follow-up visit, this proportion of patients increased to around one fifth (21.1%) by the end of the study period. This in turn means that 78.9% of the switched patients remained on the new biosimilar therapy beyond the study period. Specifically, this applied to 80.7% of psoriatic patients and slightly more than half (53.8%) of HS patients.
Of the patients with an adalimumab biosimilar who were switched back, 73.5% received the original adalimumab (Humira®) again and 26.5% were prescribed a completely different therapy with other groups of active ingredients (antibiotics, guselkumab, ixekizumab, etc.). All (100.0%) of those who were switched back to an etanercept biosimilar received the original etanercept (Enbrel®) again.
In both studies of Sigurdardottir et al. and Chan et al., switched patients were transitioned back to the original Enbrel® therapy [18, 20]. This is completely consistent with our study on Enbrel® switchbacks. Alten et al. shows that the patients who were switched back to a previous Humira® therapy also switched back to this preparation [15, 21]. All in all, there is a tendency that the few patients who experienced a switch back were generally switched back to their previous form of therapy. Treatment with other active ingredients or completely different treatment options were not discussed or shown.
This real-world data shows that biosimilar therapy is successful in around 80% of the observed population. Even though the patient groups were very different in size (181 psoriatic/PS vs. 13 HS patients), changes in treatment had better overall results for the PS patients – in terms of the skin symptoms – than for the HS group. As far as the joint symptoms were concerned, the percentage of patients reporting a worsening of pain was not significantly different between the HS and PS groups. In the event of worsening of symptoms or occurrence of side effects, switching back to the biologic might be necessary and prove to be problem-free.
Our study has some important limitations. It is a retrospective study with considerable differences in the sizes of the patient groups. For this reason, a thorough statistical analysis was not possible. As a retrospective study relying on clinic attendance and self-reporting, potential selection and recall biases are inherent limitations. Free text in the outpatient notes, incomplete scores or patients who did not attend follow-up visits or did not complete symptom reports could have introduced bias as well.
As there are still no published studies focusing on both PS and HS patients simultaneously, it would be insightful to examine the data in greater detail in the future with the aim of confirming the findings in a larger prospective study.








