Introduction
Hidradenitis suppurativa (HS) is a chronic skin disease characterized by highly recurrent abscesses. HS is an inflammatory dermatosis involving the sebaceous and apocrine glands [1]. The incidence of the disease is 6 per 100,000 per year and can affect up to 1% of the European population [2]. HS has a significant impact on the health-related quality of life both physically and mentally. Considering the pain, discharge, smell and associated pruritus, HS has been documented to have a negative influence on patients’ health-related quality of life [3]. Moreover, the disease has often been correlated with severe socio-economic consequences, a higher incidence of depression, and the fear of stigmatization. All of these factors can lead to suicide. Patients with HS have a higher risk of suicidal tendencies than the general population [3]. The clinical characteristics of hidradenitis suppurativa vary from rare, benign inflammatory nodules to extensive abscesses, sinus tracts with scarring. The inflammatory reaction leads to the abscesses extending deep into the subcutaneous tissue and then developing intercellular sinus tracts, resulting in irregular scars [4, 5]. Treatment with antibiotics, steroids and new biologic drugs, and new therapies like small molecules (JAK-inhibitors) have their limitations. Pharmacological treatment does not produce lasting treatment effects. Current guidelines (HS ALLIANCE) recommend considering surgical options in severe, recurrent cases of HS that have not been controlled by systemic medications, which is in line with the recommendations of the European S1 guidelines for the management of HS [6]. Surgical treatment in advanced stages of the disease is one possible therapy. Classical surgical treatment of HS consists of: deroofing, primary closure or reconstructions with skin flaps or split thickness skin grafts [7]. Surgical treatment appears to be a very effective method that has a positive response in improving quality of life [3].
Aim
The aim of the study was to evaluate the surgical treatment of 31 patients treated surgically at the Centre for Burns Treatment in Siemianowice Śląskie, with a follow-up of 6 months.
Material and methods
In this study, we enrolled 31 patients surgically operated for hidradenitis suppurativa between 2019 and 2021 at the Centre for Burns Treatment in Siemianowice Śląskie, Poland. We included both male and female patients with hidradenitis suppurativa I–III grade in Hurley Scale. Exclusion criteria: pregnant women, breastfeeding women, cancer in the last 5 years – presence of basal cell carcinoma (BCC), current biological and pharmacological treatment for HS. Surgical treatment consisted of a wide excision of lesions located in the armpits, groin, buttocks and intimate areas. We used the basic methods of reconstructive surgery available at our centre: 1) rotation flap reconstruction, 2) autogenous skin grafts, 3) mixed treatment (split thickness skin graft and skin flap reconstruction), 4) vacuum assisted closure (VAC) and split thickness skin grafts. Each patient was observed in our General Surgery Outpatient Clinic at the Centre for Burns Treatment in Siemianowice Śląskie, Poland. We have followed up each patient for 6 months. At the beginning we collected baseline patient characteristics such as age, gender, body mass index (BMI), Hurley Stage, types of surgical interventions, comorbidities, and follow-up after 6 months in each patient.
Statistical analysis
The study project was consulted and after data collection, statistical analysis was performed. Statistical analysis was performed using Statistica 13.0 software (TIBCO Software Inc., Carlsbad, CA, USA). The Shapiro-Wilk test was used to assess the normality of the distribution. All quantitative variables had a non-normal distribution. Spearman’s rank correlation coefficient (rs) was used as a measure of the correlation. The Mann-Whitney U test and the Kruskal-Wallis test were used to analyse quantitative data. All quantitative data were reported as the median and their spread as quartile deviation (QD). Qualitative data were analysed with the χ2 test. Yates’ correction was applied for subgroups of less than ten subjects. Statistical significance was assumed at p < 0.050. For multiple comparisons, p-values were corrected using the Bonferroni correction.
Results
The basic characteristics of the surgically treated patients are attached in Table 1.
Table 1
General and clinical characteristics of the study group
There was a statistically significant (p < 0.050) positive correlation between patients’ age and BMI, disease duration and time of diagnosis. BMI value additionally correlated with disease duration and time of diagnosis, while disease duration correlated with time of diagnosis (Table 2).
Table 2
Spearman’s rank correlation coefficient (rs) between quantitative factors
| Age | BMI | Duration of disease | Time from first symptoms to final diagnosis | Time of hospitalization | |
|---|---|---|---|---|---|
| Age | – | 0.66* | 0.78* | 0.77* | 0.07 |
| BMI | 0.66* | – | 0.61* | 0.59* | –0.09 |
| Duration of disease | 0.78 | 0.61 | – | 0.95* | 0.10 |
| Time from first symptoms to final diagnosis | 0.77* | 0.59* | 0.95* | – | 0.19 |
| Time of hospitalization | 0.07 | –0.09 | 0.10 | 0.19 | – |
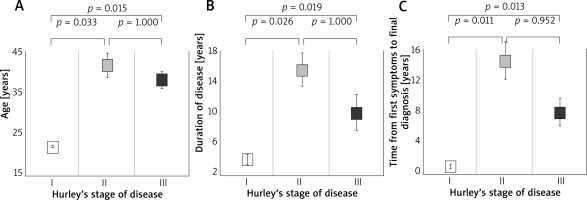
There were no statistically significant differences between men and women in parameters such as age (p = 0.439), BMI (p = 0.818), disease duration (p = 0.180), time of HS diagnosis (p = 0.224) and time of hospitalization (p = 0.851) (Figure 1).
Figure 1
Age (A), duration of disease (B) and time from first symptoms to final diagnosis (C) in patients with different grades of hidradenitis suppurativa according to Hurley’s stage system
Overweight/obese subjects (BMI ≥ 25 kg/m2), relative to normal weight subjects, were of significantly older age (38.00 ±4.50 vs. 21.00 ±1.00, p = 0.004) and had longer disease duration (10.00 ±4.50 vs. 5.00 ±1.25, p = 0.022).
Those with obesity (BMI ≥ 30 kg/m2), relative to those without obesity, were of significantly older age (42.00 ±3.50 vs. 27.50 ±6.75, p = 0.002), longer disease duration (14.00 ±3.00 vs. 6.00 ±1.25, p < 0.001) and longer time to diagnosis (12.00 ±3.00 vs. 4.00 ±2.00, p < 0.001).
Patients with metabolic syndrome had a statistically longer duration of disease relative to patients without metabolic syndrome (15.00 ±5.00 vs. 7.00 ±4.00, p = 0.048).
Factors that differentiated patients in each Hurley Stage were age (p = 0.011), disease duration (p = 0.011) and time of diagnosis (p = 0.005). Patients in class I were significantly younger than patients in Hurley Stage II (p = 0.026) and Hurley Stage III (p = 0.019). Age did not differentiate between Hurley Stage II and III patients (p = 1.000). Patients in Hurley Stage I also had a shorter illness duration than patients in Hurley Stage II (p = 0.025) and III (p = 0.019) and had a shorter time to HS diagnosis than patients in Stage II (p = 0.011) and III (p = 0.013). Disease duration and time to diagnosis did not differentiate between patients in Hurley Stage II and Hurley Stage III (p = 1.000 and p = 0.952, respectively).
Evaluation of surgical treatment (6-month follow-up) showed that surgical treatment gives a very high percentage of complete wound healing after wide excisions (83.87%) using surgical reconstructive methods. The most common complications of healing are keloids (6.45%) and minor wound dehiscence during healing (6.45%). We found a recurrence in 1 patient only at 6 months’ observation in the operated site 6 month post operatively (3.23%).
Discussion
Medical treatment with topical or systemic antibiotics is widely used as the first line in the management of mild HS (Hurley I). However, in severe cases, surgical treatment should be considered. In the early stages of abscess formation, a simple incision and drainage of the abscess and decompression of the sinus tract are often performed. Drainage is not necessarily effective in the long run as it is associated with a high rate of recurrence [8]. First mentions about surgical treatment in HS date back to 1950 [9]. Multiple studies reported that a wide surgical excision is important and effective in order to prevent complications and recurrence of hidradenitis suppurativa [10]. There is a great impact on improvement of the health-related quality of life after surgical treatment in HS [3]. Meta-analysis and systemic reviews reported that there is insufficient evidence to show the advantages of flaps over skin grafts although they have superior effects to primary closure [11]. However, surgical treatment is often carried out too late, after many years of the disease, after many ineffective cycles of drug treatment [12]. Our study confirms these findings, the median time from first symptoms to accurate diagnosis was 6 years, and the median duration of the disease was 8 years. Our patients before surgical treatment had multiple ineffective antibiotic therapies.
Extensive wide resection of the entire lesioned skin, subcutaneous fat and deep fascia ensures removal of all infected apocrine glands, subcutaneous abscesses, sinuses and ducts, which guarantees the effectiveness of surgical treatment [13]. For this reason, less invasive methods like local incision and drainage of abscesses or limited excision due to the inability to perform flap surgery are likely to fail [14, 15].
A meta-analysis by van der Zee et al. found a 27% recurrence rate after deroofing, while the largest open study to date found a 17% recurrence rate [16]. In a systematic review by Mehdizadeh et al., the postoperative recurrence rate for hidradenitis suppurativa was 15% for primary closure, 8% for skin flaps and 6.0% for skin grafting [11]. Moosa et al. reported a 37% recurrence rate for the inguinal area [17]. Other studies reported recurrence rates of 0% up to 70%, and Ovadja et al. meta-analysis reports the recurrence rate of 13% [18]. There is no doubt that the recurrence depends on many factors in the patient, nevertheless, there are probably also surgical factors. Our results showed that recurrence in 6 months occurred in 1 (3.23%) patient only. However, we are aware that 6 months’ follow-up may be too short a period, but so far, we have not noted at the time of writing this paper any new HS recurrences after surgery (at the operated sites) and in some patients it has been 3 years since surgery.
Excision and split thickness skin graft (STSG) is the primary tool in surgical reconstructive treatment, and the outcome of this procedure is often satisfactory [19–21]. However, graft failure is another common problem in areas exposed to shear forces, such as the axillae, groin and buttocks [22, 23]. Soldin et al. concluded that excision of the entire hair-bearing skin and reconstruction with local skin flaps is the treatment of choice for HS [24]. The 31 patients included in this study in 2019–2021 at the Centre for Burns Treatment in Siemianowice Śląskie were operated on using classical reconstructive methods (skin grafts and local skin flaps). We prefer treatment by performing a local skin flap, although in some cases the defect cannot be easily closed by the local skin flap. In some patients, we have used VAC therapy, which we believe does an excellent job in preparing the wound for possible STSG (e.g. buttocks). In 80.65% of our studied group, we performed a skin flap surgery. In a multi-centre comparison by Ovadja et al., 95% of patients (of 107 patients) who underwent reconstruction with skin flaps were in stage 3 Hurley disease, and these patients also had the highest number of risk factors, including type II diabetes and obesity, but reconstruction with skin flaps resulted in the lowest recurrence rate. In addition, there was a significantly higher rate of complications (25% vs. 8–10% after other reconstructions) and frequent wound dehiscence (10%) [18]. Ovadja et al. presented a total of 107 surgical interventions performed on 54 patients. The overall recurrence rate was 31.8% after a median follow-up of 30 months, with a significant difference between primary closure (48%), secondary intention healing (16%), split thickness skin grafts (29%), and local fasciocutaneous flaps (10%) (p = 0.03). Surgical complications requiring reoperation occurred in 2% after primary closure, 0% after secondary intention healing, 13% after split thickness skin graft, and 15% after local fasciocutaneous flaps (p = 0.11). Our results showed that we reached complete healing in 83.87% of patients. Our most common complication was wound dehiscence and keloids (6.45%). Of course, our limitations are the small group of patients (only 31 patients). The methods we used for these patients were classical reconstructive methods (STSG and fasciocutaneous skin flaps). Improvements in surgical techniques in HS reconstructive surgery are still required. Gierek et al. performed the first co-graft acellular dermal matrix and STSG in HS [25]. These are the first reports of this novel surgical treatment for HS. Gierek et al. also used LASCA (laser speckle contrast analysis) in the method presented. A device with which it is possible to monitor the preoperative, postoperative condition and monitor wound healing. It seems that the use of new reconstructive techniques and new diagnostic methods like LASCA can significantly improve the results of surgical treatment of HS. Limitation of this study is a small group of 31 patients, which will be extended in further research accordingly. Therefore, we believe that further research should be conducted on the surgical treatment of patients with hidradenitis suppurativa.
Conclusions
HS is still an under-recognized disease as we demonstrated in this study. Systemic treatments are often ineffective and it seems that in severe cases of HS surgical treatment can have good clinical results. Surgical treatment is an effective method in HS treatment. The relatively low recurrence rate after 6 months and, in most patients, full healing, support the good therapeutic effect of surgical treatment. However, in this study we only compared standard surgeries (flaps and STSGs), thus further studies need to expand the study group and use other reconstructive methods available to treat HS.








