INTRODUCTION
Keloid scar formation results in an aberrant healing process, by excessive fibroblast proliferation, following disruption of skin integrity of varying depths, ranging from a slight scratch to a surgical incision. It gives rise to excessive scar formation with anarchic thickening, leading to the development of multinodular, firm lesions with variable coloration, ranging from erythematous to violaceous or brown pigmentation, and typically associated with pruritus and pain. The predilection sites of their occurrence are primarily the earlobes and body areas subjected to increased tension, such as the chest, shoulders, and posterior neck. However, there is no topographic discrimination and keloid can appear even in the cornea [1, 2]. Keloids can extend over a large or limited area, in single or multiple forms, agglomerated or dispersed, determining the therapeutic possibilities, particularly the surgical ones. Although keloids may not always be clinically conspicuous, they often result in cosmetic deformity and psychological burden, prompting the need for treatment that is frequently difficult to manage effectively. Many keloids are resistant to any treatment and the recurrence rate remains high after surgical excision alone ranging from 45% to 100% [3]. Postoperative radiotherapy demonstrated promising outcomes, decreasing the recurrence rate to approximately 23%. This case report aims to present a successful result in treating a recurrent giant keloid of the neck by combining extramarginal surgical excision and post-operative interstitial high-dose-rate brachytherapy. The novelty of this well-known procedure is the combined use of an immobilization device for traction relief, to avoid outcome deterioration. Through this case, while performing a literature update, the author provides an overview of the pathophysiology of keloids and emphasizes the most promising brachytherapy regimen mainly for recalcitrant ones. Furthermore, traction relief should not be neglected as an adjuvant tool for the entire radio-surgical procedure.
CASE REPORT
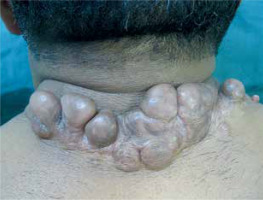
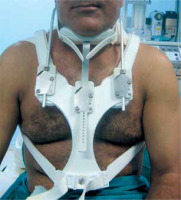
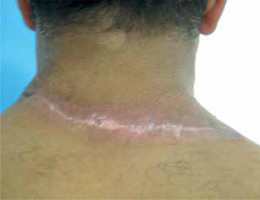
A 48-year-old man who works as a car mechanic, having a posture conducive to continuous stretching and flexion of the neck, presented to our department for a recalcitrant keloid of the back neck following surgery. It was reported that multi-layer sutures were performed adequately attempting to minimize tension forces at the wound. Despite postoperative intralesional triamcinolone acetonide injection, the keloid further expanded and became more exuberant. The initial skin injury that triggered its formation was not recognized by the patient. Physical examination revealed a recurrent keloid scar measuring approximately 14 cm × 6 cm, characterized by multiple adjacent nodular formations and pruritus and pain association (fig. 1). Under local anesthesia, an extramarginal surgical excision was performed. After meticulous hemostasis with electrocautery, subcutaneous undermining was performed along both wound margins, facilitating tension-free closure of the cutaneous defect (fig. 2). A metal catheter embedded in a plastic brachytherapy applicator-guide was placed at the dermal layer, at 5 mm depth, without kinking and extending out of the skin beyond the wound on both sides before wound closure. The closure was performed in multiple layers to achieve minimal tension (fig. 3). The closed wound, oriented parallel to the relaxed skin tension lines, was subsequently dressed with reinforced adhesive skin closure using Steri-Strips and an occlusive elastoplastic adhesive dressing to maintain a moist wound-healing environment and prevent bacterial contamination. Because of the unavoidable wide range of cervical motion, which causes continuous mechanical traction on the wound, a cervicothoracic with a cervical immobilizer was applied in the operating room after dressing and maintained for 2 months (fig. 4). The first iridium-192 high-dose-rate brachytherapy fraction of 6 Gy was administered 6 hours postoperatively, with a total dose of 30 Gy delivered in five fractions. Otherwise, histopathological findings excluded a possible diagnosis of dermatofibroma and the diagnosis of keloid was retained. At 6 months postoperatively, the scar was flattened and soft to palpation without significant enlargement, except for mild redness consistent with post-radiation radiodermatitis (fig. 5). No keloid recurrence was observed up to 18 months of follow-up. The patient was informed about the success rate and harmful effects of radiotherapy including a possible risk of late carcinogenesis, and signed informed consent forms before starting the combined surgical excision and radiation therapy procedure.
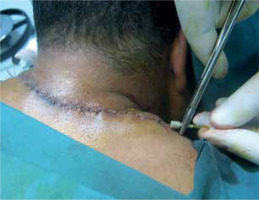
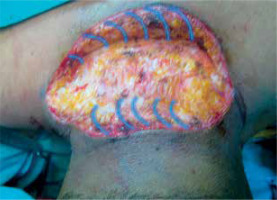
Figure 2
Cutaneous defect after keloid excision. Subcutaneous undermining along both wound edges (schematically shown as blue arches)
DISCUSSION
Keloid scar is a benign dermal fibroproliferative growth with immature collagen of a swirling nodular pattern, which is poorly vascularized with no lymphatics or elastin. Thus, keloids are characterized by exuberant, erythematous scars, which grow over time beyond the margins of the original wounds invading the surrounding normal dermis without spontaneous resolution. In contrast, hypertrophic scars rise above the original wound without overlapping the adjacent skin and typically regress after several months [4, 5]. The pathogenic mechanisms underlying this fibrotic skin condition of unknown etiology are only partially understood. The wound-healing cascade begins the moment the skin is injured. However, as for this case study, the causative event may remain unrecognized or forgotten, because the pernicious nature of keloid formation may be its appearance after a long interval or its occurrence following an insignificant skin injury such as an insect bite or a slight scratch. Although the fundamentals of the wound healing process, including hemostasis, inflammation, proliferation, and remodeling, seem to be decoded, the exact mechanism of wound healing regulation remains a mystery. Therefore, detailed knowledge about the central stimulus triggering keloid formation and key factors resulting in such scars is scarce [6, 7]. Otherwise, multifactorial mechanisms for keloid formation include dysregulated levels in growth factors such as transforming growth factor β (TGF-β) and platelet-derived growth factor (PDGF), increased collagen synthesis, tension alignment, and genetic and immunologic contributions [7]. Recent studies indicate that the microenvironment enriched in inflammatory cytokines, mainly TGF-β, interleukins, PDGF, etc., reciprocally upregulate the expression of pro-fibrotic genes leading to an aberrant wound healing process towards excessive collagen deposition and fibrosis. Increased fibroblast activation, due to over-expression of TGF-β1/2 and decreased expression of TGF-β3, leads to overproduction of the extracellular matrix component including collagen, and thus hypertrophic or keloid scar formation. Furthermore, it was suggested that a more prolonged inflammatory period, with immune cell infiltration, may contribute to increased fibroblastic activity in keloid tissue. Nevertheless, immunohistochemical investigations demonstrated a high amount of activated immune cell infiltrate in the keloid specimen excision, consisting of CD3+, CD4+, CD8+, and CD45R0 [8–10]. It has also been suggested that fibroblasts exhibit different actin filament stiffness and force generation in keloid tissue compared with normal fibroblasts, which may explain the extension of the keloid beyond the original wound margin [2]. On the other hand, skin tension over a healing wound stimulates fibroblast proliferation and increases collagen synthesis and deposition, which will most likely result in pathological scar formation and could explain the appearance of keloids in areas of increased tension such as the back of the neck where wound stretching is favored by anterolateral cervical movement [8, 9]. It appears that areas subjected to greater skin stretching and contraction are important triggers driving keloid formation. In addition, mechanical forces with high tension and cyclical stretching rather than static forces or cyclical mobility without tension are important for keloid generation and pathologic scar ingravescence. Mechanical forces strongly modulate the cellular behavior by which physical forces are converted into biochemical signals that lead to abnormal scarring [10, 11]. In addition, in vitro studies have shown that mechanotransduction mechanisms can stimulate cell proliferation and angiogenesis [12, 13]. For this reason, an immobilization device for tension relief consisting of a thoracic corset with a cervical immobilizer was applied to our patient immediately after the intervention was completed and maintained for 2 months, as the critical time for the development of a pathological trait in the wound-healing process is around 2 months after the trauma [14]. To our knowledge, the use of immediate postoperative immobilization devices to reduce skin tension has not been previously described, and we believe that this has played a major role in minimizing scar enlargement and eliminating such favorable conditions of recurrence. Moreover, the tensile forces acting on the wound area were further reduced by tensionless closure following subcutaneous undermining at each wound edge, multilayer sutures, and appropriate scar placement according to the relaxed skin tension lines. Otherwise, tension-free closure is often managed by reinforced sutures or by using plastic procedures such as Z plasty, skin grafts, and cutaneous flaps [5, 15]. However, a broad literature search revealed that such interest in tension relief through the use of immobilization devices had never been described in the therapeutic arsenal for keloids.
Nevertheless, it is difficult to predict the prognosis of abnormal scarring particularly keloid scars which are challenging and discouraging to treat due to the considerably high rate of recurrence. For this reason, combined and less aggressive procedures are usually performed since there is no consensus guideline. Therefore, the therapeutic strategy depends on the surgeon’s experience, the patient’s psychological profile, and the keloid characteristics regarding location, extent, and maturation. Postoperative adjuvant radiotherapy has been widely recognized for its effectiveness in treating keloids, particularly brachytherapy, which delivers more focused radiation to the target tissue, ensuring a significant reduction in recurrence rates and better safety for the surrounding normal tissue [16]. An international advisory panel on scar management recommended postoperative adjuvant radiotherapy which is the most efficacious treatment regimen, particularly for severe and recalcitrant keloids [3]. Nevertheless, the recurrence rate of keloids in patients who received postoperative adjuvant radiation therapy has been reported to be lower than in those receiving radiotherapy alone (22% vs. 37%), while the recurrence rate in patients who underwent surgery alone ranged from 50 to 99% [17]. Brachytherapy has been used to treat keloids since 1967 [18], and it is to date more effective particularly the interstitial brachytherapy with a high dose rate (HDR) which is the primary modality of brachytherapy. However, there is no consensus regarding the optimal radiation dose, timing, and fractionation schedule, which should be adjusted according to keloid characteristics and location. Several publications are following treatment regimens ranging from 2 to 5 sessions. The first dose should be administered early, within 24 hours, and delivering 12–20 Gy [16, 19, 20]. However, the biologically effective dose necessary for improving radiation therapy performance is not standardized. While some studies have shown that a recurrence rate below 10% is achieved when the dose exceeds 30 Gy, others have set the threshold at 20 Gy. A shorter interval between surgery and radiation therapy is important to avoid keloid recurrence [14, 21–23]. Moreover, a lower recurrence rate was observed with HDR brachytherapy (10.5%) rather than with low-dose-rate brachytherapy (21.3%) or with external beam radiation (22.2%) [24, 25]. The present case showed a successful outcome using HDR brachytherapy that started 6 hours postoperatively. In addition, the concomitant use of a relief traction device immobilized the neck, thereby preventing continuous wound tension — one of the key stimuli of fibrogenesis — and resulting in stable outcomes without scar enlargement.
The exact mechanism of radiotherapy to inhibit keloids is not clearly defined and may be various. It may prevent fibroblast repopulation and inhibit fibroblast function after surgery by further modulation of humoral or cellular factors or by accelerating fibroblast senescence and inhibiting angiogenesis. Radiation therapy can also strongly inhibit the inflammatory response by weakening immune cells’ function and reducing dysfunctional blood vessel formation [17, 26]. Hence, there is substantial evidence that radiotherapy suppresses fibroblast proliferation and induces apoptosis within keloid tissue.
Despite the effectiveness of radiation therapy, it remains underutilized out of concern for the possible development of secondary malignancies. However, a potential carcinogenic risk in patients presenting with keloids regardless of radiation therapy should be considered [10]. A correlation between keloid formation and the occurrence of specific malignancies, notably skin and pancreatic cancers, has been reported in Asian population. The overall risk of skin cancer was 1.73-fold higher in patients with keloids. On the other hand, the risk of carcinogenesis related to keloid radiation therapy is extremely low due to the advances in radiation technology [27]. Likewise, a survey conducted in 1996 among 508 institutes of therapeutic radiology and oncology concerning radiotherapy and benign pathology such as keloids, showed obviousness in preventing keloid formation using radiotherapy which is the most widely accepted indication [28]. Otherwise, telangiectasia, skin atrophy, hyperpigmentation, and subcutaneous fibrosis are the most frequent keloid radiation side effects [29].
CONCLUSIONS
At present, keloid management remains a multimodal approach with unpredictable outcomes. According to the most recent data, and as corroborated by the present case, postoperative adjuvant HDR interstitial brachytherapy initiated within 24 hours after extramarginal excision represents a promising treatment modality for keloids, yielding favorable outcomes. However, interstitial catheter placement requires complete closure of the cutaneous defect. Furthermore, more studies of a prospective, randomized nature are needed to standardize its use. The author highlights the crucial and synergistic effect of relieving wound traction using an immobilization device for all areas with unavoidable mobility. This adjuvant procedure has not been previously reported and can ensure success and prevent outcomes from worsening. Therefore, elucidating the mechanobiological environment of wound healing may facilitate the development of innovative strategies for scar prevention and therapy, including modulation of mechanoreceptor and nociceptor activity, suppression of inflammation, and regulation of cell proliferation and angiogenesis.