Introduction
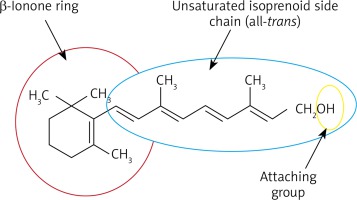
According to the IUPAC (International Union of Pure and Applied Chemistry) and IUBMB (International Union of Biochemistry and Molecular Biology), retinoids are compounds containing four isoprene units with a head-to-tail structure [1]. Retinol, retinoic aldehyde and retinoic acid belong to retinoids with a non-aromatic fragment of β-ionone in their molecule. The term “retinoid” refers to the synthetic and natural analogues of vitamin A. Retinoids are a class of compounds derived from vitamin A or showing structural and/or functional similarities to vitamin A. According to the latter definition, retinoids are molecules that can bind to and activate the appropriate nuclear receptors and to induce transcription of relevant genes either directly or after metabolic transformation [2]. Retinoids are widely applied in cosmetics being a potent dermatological agent used in acne, psoriasis as well as other skin diseases.
The objective of this study is to introduce and compare different types of retinoid uses in cosmetic and dermatological treatments. Moreover, this paper should address the issue of the cellular activity of retinoids.
Retinoids are compounds of both natural, biologically active forms of vitamin A (retinol, retinal and retinoic acid) as well as synthetic analogues of retinol (Figures 1, 2). Synthetic analogues have a benzene ring instead of cyclohexane (etretinate, acitretin, tazarotene). Based on the molecular structure and properties, retinoids can be divided into three generations:
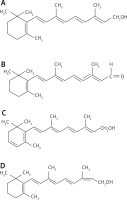
Figure 2
Structural formulas of selected retinoids: retinol (A), retinal (B), 3-dehydroretiol (vitamin A2) (C), 13-cis-retinol (D)
First generation – natural retinoids, monoaromatic compounds obtained by modifying polar groups at the end and side chain of the polyene vitamin that do not act selectively – retinol (vitamin A) and its metabolites – retinal, tretinoin, isotretinoin,
Second generation – monoaromatic retinoids, synthetic compounds in which the cyclohexene ring is replaced by a benzene ring; synthetic analogues of vitamin A (etretinate, acitretin),
Third generation – polyaromatic retinoids formed as a result of cyclization of polyene side chain and characterized by selective activity towards receptor (arotinoid, adapalene, tazarotene) [3].
Retinol, retinal and retinoic acid have the same biological features as vitamin A. Retinoids are involved in the process of embryogenesis during development of the nervous system, liver, heart, kidneys, intestine, eyes and limbs. Retinoids are used in treatment of the so-called “night blindness” because they are responsible for proper functioning of the organ of sight. They are associated with formation of rhodopsin. They are used in pharmacotherapy of diseases such as acne and rosacea, psoriasis, cancer, inflammation of hair follicles with bacterial aetiology, pyoderma, lupus erythematosus and ichthyosis. Retinol does not exert a significant biological effect on tissues but becomes active after transformation into more active metabolites, the most important one being the retinoic acid characterized by its multilateral action. Retinoic acid (RA), occurs in the form of two isomers: the fully-trans form and the 9-cis form that affects proliferation and differentiation of cells by regulating the respective genes. Retinoids are involved in diverse biological activities including cellular growth, cellular cohesion, immunomodulatory effects, and anti-tumour functions.
Vitamin A and its derivatives, particularly retinol, are substances slowing the aging process most effectively. Fat soluble retinol penetrates the stratum corneum and it slightly penetrates into the dermis. When retinol reaches a keratinocyte, it enters its interior and binds to an appropriate receptor. There are four groups of receptors with high affinity towards retinol (CRBP) [4, 5]. Retinol stimulates the cellular activity of keratinocytes, fibroblasts, melanocytes and Langerhans cells. Retinol, by interacting with receptors inside keratinocytes, promotes their proliferation, strengthens the epidermal protective function, reduces transepidermal water loss, protects collagen against degradation and inhibits the activity of metalloproteinases which are responsible for degradation of the extracellular matrix. Moreover, it enhances remodelling of reticular fibres and stimulates angiogenesis in the papillary layer of the dermis. Irritant properties of vitamin A and its derivatives as well as their instability are factors that limit their application in cosmetic and pharmaceutical products [6].
Retinoids: a mode of action
Retinoids, as compounds that are sparingly soluble in body fluids (lipophilic compounds), need specialized proteins to transport them (complex with Transthyretin – (prealbumin) is a retinol binding protein (vitamin A). Results of the study by Hyung et al. proved new applications of RBP and retinoids as stabilizers of transthyretin [7]. These are proteins such as RBP and CRBP. Cytosolic retinol binding protein (CRBP), which is present in cytoplasm, shows affinity for retinol, while cytosolic retinoic acid binding protein (CRABP) has affinity for retinoid acid. There are two subtypes of both groups of receptors: CRBP I and II and CRABP I and II. Intracellular concentration of retinoids depends on their binding to cellular CRABP I and II. Studies show that CRABP II (it is the main form present in the epidermis) is much more abundant in the skin than CRABP I (modulates the level of retinoic acid in different tissues) [8]. These proteins activate appropriate nuclear receptors, thanks to which retinoids exert their biological effect on particular tissues, organs and cells. Retinoid nuclear receptors (RNR, which represent a steroid thyroid hormone receptor) include:
RA receptors (RAR), its natural ligand is retinoic acid (RA), and
Retinoid X Receptors (RXR), its natural ligand is 9-cis-retinoic acid.
Within these receptors, there are three types of isotypes: α, β and g (RARα, RARβ, RARγ). They may be further divided into isoforms. The human skin mainly contains RXRg and RARα. Retinoids activate receptors in the form of dimers which in turn bind to the appropriate RARE element, i.e. the domain of the DNA response. They are located near the gene promoter sequences regulated by retinoids. Receptor expression is not regular and is described in only some tissues and organs, including the epidermis, dermis, sebaceous glands and hair follicles, or in cells of the immune system.
Vitamin A and its derivatives are involved in embryogenesis. Retinoids take part in development of the nervous system, liver, heart, kidneys, intestine, eyes and limbs. Two-step oxidation occurring in the target organ cells results in conversion of retinol to its active form – retinoic acid. After entering the cell, retinol dehydrogenase (RDH) or alcohol dehydrogenase (ADH) catalyse the oxidation of retinol to retinal. This reaction may be reversed by the same enzyme because oxidation of retinol to retinoic aldehyde is a reversible process. Moreover, many enzymes can catalyse the reverse reaction, i.e. the conversion from retinamide to retinol. It indicates the presence of an additional mechanism which regulates the local retinol concentration in the tissues [4]. Subsequently, retinol is oxidized to retinoid acid by retinaldehyde dehydrogenase (RALDH) or some enzymes of the CYP family (belonging to the cytochrome P450 family). This reaction is irreversible; the product formed is a natural ligand of nuclear receptors and it reflects the activity of vitamin A. Further oxidation of the retinoic acid by CYP26 enzyme results in obtaining inactive vitamin A metabolites.
Vitamin A and its derivatives, particularly retinol, are among the most effective substances delaying the process of aging. Fat-soluble retinol penetrates into the stratum corneum and, to a small extent, into the dermis. It is important to increase penetration of retinol, thus increasing its spectrum of activity, and to control a potential action in laboratory tests, and then to enhance the procedure effectiveness. Retinol, after reaching keratinocyte, penetrates into its interior and binds to an appropriate receptor. Cytosolic retinol binding protein receptors show high affinity for retinol [5, 6]. In epidermis, retinoids may influence secretion of transcription and growth factors. They are responsible for proliferation of the living layer of the epidermis, strengthening of the protective function of epidermis and reduction in excessive transepidermal water loss (TEWL). Moreover, retinoids protect against degradation of collagen and inhibit activity of metalloproteinases, enhances angiogenesis in the papillary layer of the dermis [9, 10]. The irritant effect of vitamin A and its derivatives and their instability are factors limiting their use in cosmetic and pharmaceutical products. Intracellular penetration is the main way of transport during which molecules move through the intercellular cement structure composed of ceramides, sterols, phospholipids and fatty acids. Intercellular cement has a lamellar structure, the lipid layer and hydrophilic layer are arranged alternately [8, 11]. Further studies on retinol activity in various cosmetic formulas are required in order to select the one that is best tolerated by the skin and to determine whether the concentration significantly influences the effect it exerts on the skin. Natural retinoids have a positive effect on the skin parameters. They are characterized by good absorbability (they are fat-soluble) which improves the skin function. Retinoids boost production of epidermal proteins and accelerate the process of keratinization, forming a layer of keratin which is more developed. Retinol penetrates into the basal layer of the epidermis (composed of living (nucleated) cells that are constantly producing new cells) as well as to a small extent, into the dermis and marginally to the subcutaneous tissue. In the case of retinol applied topically, there is an interaction with specific nuclear receptors. Retinol makes the connections between epidermal cells more loose and facilitates keratosis. What is more, it enhances epidermis turn-over and accelerates proliferation of the basal layer of epidermal cells and the stratum corneum. In keratinocytes, proliferation AP-1 transcription factor, exposed to various stimulants, growth factors and cytokines, plays a major role. In retinol-treated aged human skin, AP-1 complex is comprised of c-Jun/c-fos and c-Jun transcription factor was increased [12]. Due to the fact that retinoids exert anticomedogenic effects, they regulate the process of shedding within sebaceous glands ducts. What is most important, retinoids decrease activity of enzymes participating in lipogenesis and block differentiation and cellular divisions of sebocytes [12]. Moreover, they reduce discoloration of the skin, reduce its pigmentation by about 60% and contribute to a proper distribution of melanin in the skin. Topically applied retinoids also influence the function of melanocytes, providing regular arrangement of melanin in the epidermis. They also block transport of melanin to epidermal cells and diminish the activity of stimulated melanocytes. An increase in synthesis and activity of tyrosinase, disturbances in subsequent steps of melanogenesis or a decrease in the amount of melanocytes is related to inhibition of melanogenesis. Retinoids are also commonly known as biologically active anti-aging molecules. Retinol stimulates fibroblasts to synthesize collagen fibres (stimulates the activity of fibroblasts and increases their number), improves skin elasticity (removes degenerated elastin fibers) and promotes angiogenesis [13]. Some studies indicate that retinol also enhances production of elastin fibres [14]. Moreover, retinol inhibits matrix metalloproteinases (MMPs) and enhances synthesis of tissue inhibitors of metalloproteinases (TIMPs) [15]. Changes within collagen and elastin fibres are associated with photoaging. It leads to occurrence of wrinkles and loss of the skin firmness and elasticity. Collagen fibres atrophy is caused by an increased expression of collagenases (MMP-1), gelatinases (MMP-2) and stromelysin-1 [16] as well as enhanced expression of elastase and MMP-9 associated with degradation of elastin fibres. Retinol counteracts development of precancerous conditions as a result of hampering the activity of atypical cells, which has been proved by the results of studies [17]. ECM-producing cells in the skin are activated by retinol and cause its production in the aged skin. Activation of fibroblast production is stimulated through TGF-β/CTGF pathway. Connective tissue growth factor (CTGF) including immunostaining of TGF-β1, which is the regulator of ECM homeostasis, is increased by retinol [18]. By reducing the amount of sebum secreted by the skin, retinoids reduce the tendency to form blackheads [19]. Excessive degradation of the stratum corneum and keratosis of hair follicles is associated with vitamin A deficiency. Regulation of secretion within sebaceous gland ducts make retinoids produce an anticomedogenic effect. Retinoids decrease the activity of enzymes participating in lipogenesis. Moreover, they block cellular division of sebocytes and differentiation [20]. They are widely used externally in the treatment of acne, psoriasis, excessive dryness, skin keratosis and hair and nail disorders [13, 20].
Application in cosmetology and dermatology (Table 1)
Tretinoin (all trans-retinoic acid) is the most bioactive form among retinoids applied topically to the skin. Tretinoin increases the epidermal cellular turnover, it also causes dispersion of melanin granules. Tretinoin’s inhibition of MMPs results from blocking AP-1, not upregulating the tissue inhibitor of MMPs (TIMP1). The most commonly used tretinoin concentration in anti-acne therapy varies from 0.01% to 0.4%. It comes in the form of gel or cream applied topically. Retinoic acid may have different formulas: gel (0.01%, 0.25%), cream (0.025%, 0.05%, 0.1%), new technology microspheres (0.04%, 0.1%), solution (0.05%), and emollient (0.05%) [21, 22].
Table 1
Retinoid uses in cosmetic and dermatological skin care treatments
Retinol is most frequently used in cosmeceutical treatment. It is very stable in product formulations and well tolerated. It provides better effects than retinoic acid applied in equivalent doses. Retinoic acid proves to be approximately 20 times more powerful than retinol. Firstly, retinol is converted to retinoic acid through a two-step oxidation process. Retinol has an ability to bind to the retinoic acid receptors. The process begins when free retinol is combined with a specific cytoplasmic protein that binds retinol. The resultant complex is a substrate for retinol dehydrogenase, an enzyme that catalyses the conversion of retinol to retinaldehyde. Retinaldehyde is oxidized to retinoic acid by retinaldehyde oxidase [23]. Retinol is known to be a molecule which improves the skin texture, dyspigmentation, dryness, and fine lines. The optimal concentration to balance the skin irritation against effectiveness has not been determined. Retinol concentration in the cosmetic product is between 0.0015% and 0.3% [24].
Retinal is the aldehyde formulation of vitamin A, i.e. the oxidized form of retinol. Retinal is used in cosmeceuticals, however, its efficacy in the skin treatment is limited. Similarly to retinyl esters, it is a stable derivative of vitamin A but it only mildly improves wrinkles and the skin texture. As compared to retinoic acid, it is less irritating and well tolerated. It is used to improve signs of photoaging [25].
Retinyl esters, such as retinyl acetate and palmitate, are commonly used in cosmeceuticals. They are very stable but first they need to be converted to retinol by cleavage of the ester bond, and in the subsequent stage into retinoic acid. It results in decreased effectiveness of anti-wrinkle properties (smaller increase in the epidermal thickness) as compared to retinol and retinoic acid [26].
Adapalene is a naphthalenecarboxylic acid derivative with a retinoid-like activity. As a result of intracellular association with nuclear receptors of retinoic acid, it changes gene expression and mRNA synthesis. It is a strong modulator of keratinization of hair follicle cells, moreover, it modifies keratinocyte metabolism, increases proliferation, and thus exerts a keratolytic effect [20].
Tazarotene, approved by the US Food and Drug Administration, is a synthetic retinoid (prodrug). It is applied in topical treatment of plaque psoriasis and acne vulgaris (AV). Tazarotene is also used in adjunctive treatment of specific clinical manifestations of chronically photodamaged skin (hyperpigmentation and hypopigmentation as well as facial fine wrinkling and benign facial lentigines). Topical tazarotene is used in concentration of 0.05% to 0.1% [27].
There are numerous publications on efficacy of various derivatives of vitamin A used in treatment of juvenile acne, acne vulgaris and other types of acne as well as in treatment of diseases related to keratosis disorders, the so-called ichthyosis and psoriasis [3]. There are reports describing effects of skin care preparations containing maximum 0.3% of retinol on the skin condition. The most recent scientific articles on retinol describe the combination of 4% hydroquinone with 1% retinol in a 24-week therapy of the skin with sun damage (photoaging) and melasma [27] and combinations of lyophilized retinoic acid and hydroquinone used in treatment of melasma. The lyophilized form of the acid is used to increase penetration efficiency in the case of a sensitive skin [28]. Scientists from the Johnson & Johnson Skin Research Centre reported that a stabilized form of retinol stimulated the synthesis of hyaluronic acid in the skin and influenced the expression of genes stimulating the synthesis of macromolecules [29]. Retinol in the form of retinyl palmitate, retinal and β-carotene is most commonly used in cosmetics [30, 31]. Recent studies of Kim et al. focus on the low stability of retinol in cosmetic formulas (due to its sensitivity to light, temperature, etc.). They analysed triple encapsulated emulsions with retinol containing polycaprolactone, lecithin and silica and five biomimetic cosmetic emulsions O/W in order to find solutions against the decomposition of retinol. The results of their study have confirmed that retinol stability depends not only on the temperature, but also on the type of substrate used and the method of emulsion preparation.
Conclusions
Retinol and their active metabolites such as retinal, tretinoin, isotretinoin and alitretinoin belong to a group of first-generation retinoids. Retinol has the ability to effectively penetrate the stratum corneum (lipophilic nature of retinoids). Age, cellular metabolism, cardiovascular function, stratum corneum thickness, level of hydration and analysed area of the face are important factors in mature skin therapies. The number of scientific reports on the activity of retinoids was the reason for this study.