Introduction
Treponema pallidum, human immunodeficiency virus (HIV), hepatitis B and C virus (HCV and HBV) infections are sexually transmitted diseases and all remain major health issues in the world. China has become the first. Previous studies reported about 587,464 new syphilis patients in China in 2019, and the syphilis incidence reported approximately 38.37 per 100,000 [1]. Meanwhile, the estimated number of people living with human immunodeficiency virus (PLHIV) in China has risen over 1.25 million [2]. Moreover, China has the largest prevalence of HBV and HCV worldwide, accounting for one-third of the world’s infected population [3].
Since they share the same route of transmission, co-infection is common and they affect each other in several ways [4]. Increasing morbidity and mortality have been reported in a remarkable number of co-infected patients [4]. Karp et al. found that active syphilis rises HIV acquisition risk and influences the immunologic response among PLHIV, HIV can alter the natural course of syphilis and usually results in a more malignant course [5]. In addition, HIV/HBV or HCV infection can accelerate hepatitis progression towards end-stage liver disease and affect the clinical steps of HIV [4]. Therefore, there is a great need to screen for HIV, HBV, HBC and syphilis co-infected cases.
Most published articles emphasized co-infections with HBV, HCV and syphilis in HIV cases [6–8]. However, few research studies examines co-infections and risk factors for HIV and hepatitis in syphilis patients. As one of the most developed cities in China, Shanghai is facing a huge challenge in the fight against sexually-transmitted diseases (STDs) [9].
Aim
The aim of this study was to evaluate the risk factors and incidence for HBV, HCV and HIV among syphilis patients in Shanghai, China.
Material and methods
Data collection
We conducted this retrospective cohort study at the Department of Sexually Transmitted Disease, Center of Infectious Skin Diseases, Shanghai Skin Disease Hospital Hospital from January 2012 to May 2024. A total of 8,499 patients with syphilis were enrolled. Patients’ medical records were used to collect demographic information and laboratory test results.
Inclusion and exclusion criteria
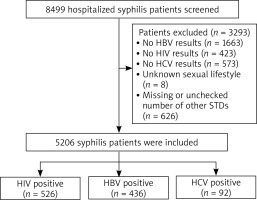
The diagnostic criteria of primary, secondary, latent, serofast and undetermined status of syphilis were published in the previous research study [10]. 1,663 patients without HBsAg test results, 423 patients without HIV test results, 573 patients without HCV Ab test results, 8 patients of undetermined sexual orientation, and 626 patients without relevant other STD test results were excluded from this study. Ultimately, 5,206 syphilis patients were enrolled in the study (Figure 1).
Statistical analysis
We used SPSS version 22.0 for data analysis. The median and interquartile range were used for presenting variables. Fisher’s exact test and c2 test were used to compare the groups. Wilcoxon rank sum test was performed for continuous variables. A binary logistic regression was applied to determine risk factors for co-infection with hepatitis and HIV among patients with syphilis. Probability (p) values < 0.05 were considered statistically significant.
Results
Of the 5,206 syphilis patients enrolled in this study (Table 1), 3,401 (65.3%) patients were men, with a male/female ratio of 1.88 : 1. The median age of the study subjects was 51 years old. 590 (11.3%) subjects had primary or secondary syphilis, 455 (8.7%) had latent syphilis, 2,287 (43.9%) had syphilis with undetermined stage, 1,874 (36%) had the serofast status. Of all those patients, there were 1,220 (23.4%) asymptomatic neurosyphilis patients, 792 (15.2%) symptomatic neurosyphilis patients, 213 (4.1%) ocular syphilis patients, 56 (1.1%) cardiovascular syphilis patients, 6 (0.1%) otosyphilis patients. 2,380 (45.8%) patients had STD co-infection. Also there were 1,722 cases co-infected with HSV-2 infections, 40 cases of gonococcal infection, 150 cases of chlamydia, and 471 HPV infections.
Table 1
Demographic and laboratory characteristics of 5,206 syphilis patients
Table 1 showed that the prevalence of HBV, HCV and HIV among syphilis patients in our study was 8.4%, 1.8%, and 10.1%, respectively. In a univariate analysis, compared with the HBV-negative syphilis group, the median age was significantly lower in HBV/syphilis co-infected group (47.5 with IQR 36–58 vs. 52 with IQR 36–61), the majority of co-infected individuals were male (251, 57.6% vs. 185, 42.4%). The rates of HCV, gonococcal and herpes virus infection were significantly higher in patients co-infected with HBV (p < 0.05). Logistic regression analysis showed that co-infected with patients co-infected with HCV (AOR = 4.1; 95% CI: 2.189–-7.77), and combined with gonococcal infection and herpes virus type II infection (AOR = 4.9; 95% CI: 2.29–10.47) (AOR = 1.354; 95% CI: 1.057–1.721) were independently associated with HBV co-infection in syphilis (Table 2).
Table 2
Multivariate logistic regression analysis for risk factors for HBV co-infection in patients with syphilis
Co-infection with HIV was found in 10.1% (526/5206) of tested subjects. Logistic regression analysis showed that male gender (AOR = 7.093; 95% CI: 3.569–14.095), age below 30 years (AOR = 6.3; 95% CI: 2.95–13.45), age group of 31–44-years old (AOR = 6.39; 95% CI: 3.151–12.99), co-infection with HSV-2 (AOR = 2.37; 95% CI: 1.621–3.465), patients who were never married (AOR = 2.439; 95% CI: 1.554–3.827) and divorced patients (AOR = 3.123; 95% CI: 1.678–5.182), and having a RPR titre of ≥ 1 : 32 (AOR = 2.126; 95% CI: 1.262–3.584) were independently associated with HIV co-infection (Table 3).
Table 3
Multivariate logistic regression analysis for risk factors for HIV co-infection in patients with syphilis
Discussion
To date, co-infection between STDs has been common and currently remains a worldwide public health problem [11].
Because STDs are affected in many ways, early diagnosis and treatment of co-infections can prevent the disease from spreading more quickly and reduce the risk of transmission to couples and children [4]. The number of syphilis cases had already seen a sharp increase over the past 20 years in China [1]. Therefore, it is an essential public health matter to evaluate the rate of co-infections with other STDs in a suitable way among the population. In this study, we focused on investigating the epidemiological features and risk factors of HBV, HCV, and HIV infection among syphilis patients in Shanghai, where the syphilis and STDs burden is the greatest in eastern China.
HBV infection is a significant public health challenge, a modelling study showed that the prevalence of HBsAg was 6.1% (5.5–6.9%) in 2016 in mainland China. In our study, we observed that the prevalence of HBV was 8.4% among syphilis patients, which is slightly higher than in the Chinese population. Meanwhile, comparing with the estimated prevalence of HCV (1.0–2.9%) [12–14] in the general population, the rate of HCV infection was reported as 1.8% of syphilis patients in the study, specifying that syphilis may not elevate the prevalence of HCV. Nevertheless, we found that HCV co-infection was still a high risk factor for HBV infection among syphilis patients. The phenomenon might be due to a dysfunctional immune system or poor HBV vaccination response and low anti-HBs antibody levels caused by chronic HCV or Treponema pallidum infection [15, 16].
Our study demonstrated that male patients and homosexuals were more associated to be co-infected with HIV. There have already been similar results of gender disparity in HIV co-infection with syphilis [17], whereas the prevalence was observed to be higher in men especially in the men who have sex with men (MSM) population. This is consistent with our conclusions. Besides, our study also revealed that younger age (< 45 years) was an independent factor for co-infection with HIV in syphilis patients. Being more sexually active, condoms neglecting, having numerous partners or more unsafe sex may contribute to a greater risk of STDs and HIV among younger people [17]. Thus, there is a vital need to develop STD education for younger populations.
Many previous reports revealed that MSM and people living with HIV may be at increased risk of HBV and HCV infection. Previous research studies showed that the rate of HCV and HBsAg in the HIV patients was greater than in the general population [18–20]. Nevertheless, other research studies showed that in HIV patients, the HBsAg rate was marginally lower than in the national general population [21]. In the current study, we found that co-infection with HIV or homosexuality among syphilis patients had no significant impact on the prevalence of HBV or HCV. The causes of the inconsistency can relate to different factors like the area, age, time of investigation, ethnicity and transmission routes. Although the present study investigated the prevalence of other infectious diseases in these patients with a significant sample size, but it is not compatible with the comparison of other published studies, it had a more comprehensive view of these diseases, and meta-analysis studies are suitable for a better understanding of this issue as for example a meta-analysis study by Marseille et al. reported association between HBV syphilis infection [22] or Jansen et al.’s study showed that 55.3% of HIV patients were co-infected with one of the HCV, HBV, or syphilis [23]. Also Hui et al.’s study showed that male gender and living in Southern China is significant risk factor for co-infection with other STDs in syphilis patients. Although the place of residence was examined in the current study, the gender of the patients was significant as shown in the study of Hui et al.
There are a number of limitations in the current study. For associations reported here, it is not known whether factors associated with HBV/T. pallidum co-infection were present before subjects were exposed to HBV/T. pallidum co-infection or became co-infected with HBV/T. pallidum. Therefore, no causal associations may be inferred. Additionally, this study is based on data from just one hospital and nearly 38.7% (3,293/8,499) of the cases were excluded from multivariate analysis because of missing data, raising the concern of selection bias. Moreover, this research was conducted in an extremely endemic area for both syphilis and HBV/HIV, therefore more studies are needed to discover the HBV and HIV co-infection in syphilis populations with more different area.