Pemphigus erythematosus, also known as Senear-Usher syndrome, is an autoimmune skin blistering disorder with an overlapping clinical presentation of pemphigus foliaceus and lupus erythematosus (Table 1) [1, 2]. Patients with classic pemphigus erythematosus present with flaccid vesicles or blisters turning easily into crusted erosions covered with piling-up scale. Lesions are typically located on the face, especially the bridge of the nose and malar area in a butterfly distribution. They may also affect the preauricular region [3]. The oral mucosa, throat, and vulva typically exhibit no signs of involvement [2]. The onset and progression of pemphigus erythematosus are typically slow. Although the distribution of the pemphigus erythematosus lesions should suggest induction by sunlight, the patient may be unaware of the photosensitive nature of the disorder.
Table 1
Overlapping clinical presentation of lupus erythematosus and pemphigus foliaceus observed in our case
In the course of the disease, secondary infection may occur, causing impetigo, healing with discoloration, and scarring. With extensive skin involvement, patients with pemphigus erythematosus may exhibit exfoliative erythroderma usually without mucosal involvement. Patients with pemphigus develop an autoimmune response directed against desmosomes, individuals with pemphigus foliaceus develop autoantibodies against Dsg 1, since pemphigus erythematosus is considered a benign variant of pemphigus foliaceus, it is expected that these patients would develop autoantibodies only against Dsg 1, however, a study conducted by Pérez-Pérez et al. demonstrate that patients with Senear-Usher syndrome may simultaneously develop antibodies against desmoglein 1 and 3. The authors hypothesized that pemphigus erythematosus is a multiple autoimmune disease [2]. The binding of autoantibodies is postulated to result in a cascade of biochemical intracellular events that lead to the loss of desmosome function. Additionally, certain HLA haplotypes (A10 or A26, DRW6) are thought to be associated, suggesting a genetic predisposition [4, 5]. The epidemiology of pemphigus erythematosus is unknown; patients with pemphigus erythematosus comprise only a small subgroup of those with pemphigus. Kumar et al., in a 2008 article, reported a high prevalence (4.4 cases per million population) [6]. However, in northern Finland, pemphigus foliaceus and pemphigus erythematosus are the most common pemphigus subtypes [7].
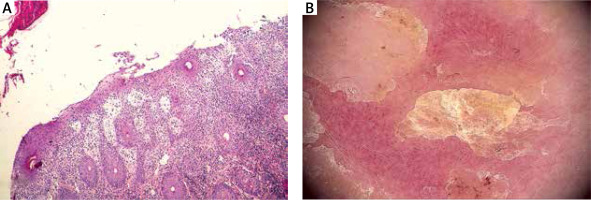
Herein, we present the case of a 5-year-old girl who exhibited mildly scaling and sharply demarcated erythematous patches and erosions on her nose and malar regions, reflecting the characteristic butterfly pattern commonly observed in cases of lupus erythematosus (as depicted in Figure 1 A). The oral mucosa, pharynx, and vulva were not involved. Skin changes had been present for 6 months, the majority of the exacerbations occurred in July and August and typically 48 to 72 h following exposure to sunlight. Dermatoscopy examination without immersion revealed irregular erythematous lesions and erosions covered with a serum crust and a thin scale, indicating the superficial character of epidermal detachment and desquamation (Figure 2 B). The background exhibited a predominant presence of a dotted vascular pattern. The dermatoscopy picture was highly suggestive of desquamative or acantholytic disease. Similarly-looking dermoscopical pictures have been described in autoimmune bullous disease [8]. The patient’s ancillary investigations revealed a positive antinuclear antibody titre (ANA 1 : 320) with specificity for PM-Scl. ELISA identified negative values of anti-ds DNA, anti-Dsg 1 and anti-Dsg 3 auto-antibodies. Chest X-ray, ECG, urinalysis were normal. The patient showed no discernible SLE organ system involvement pattern. The histopathological examination revealed fragmented hyperplastic epidermis inflamed with neutrophils and lymphocytes, crusted, and desquamative in its upper layer. Roundish acantholytic keratinocytes were subtle and visible within the granular layer. Inflammatory cells such as lymphocytes and neutrophils broadly entered the epidermis with accompanying epidermal spongiosis and oedema of the papillary dermis with dilated blood vessels. Heavy perivascular and periadnexal mixed inflammatory infiltrates were seen underneath, not corresponding to superficial acantholytic epidermal changes, suggesting that an additional underlying inflammatory process could be present. This process was highly suggestive of lupus erythematosus-like changes (Figure 2 A). Verification of the abovementioned findings in a direct immunofluorescent study for pemphigus erythematosus was not possible to perform since parents refused to take an additional perilesional punch biopsy from their daughter. Pemphigus erythematosus became a working diagnosis in this 5-year-old girl, taking into consideration her medical history, clinical-pathological correlation and dermatoscopic findings.
Figure 1
A – 5-year-old girl presents erythematous patches and scaly erosions on her face prominence resembling the butterfly distribution. These lesions had been present for over 6 months and did not respond to topical treatment. The colour of the gentian violet is marked. B – The patient presents resolved skin lesions with residual erythema following 2-month treatment with hydroxychloroquine 200 mg/day
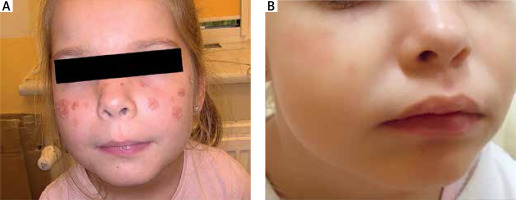
Figure 2
A – Histopathological presentation. Inflamed hyperplastic epidermis presents superficial desquamation with rounded acantholytic keratinocytes within the granular layer mixed with serum and inflammatory cells, particularly neutrophils. There is papillary dermal oedema, dense perivascular and periadnexal mixed inflammatory infiltrate with cells exocytosis into the epidermis and hair epithelia, suggesting underlying lupus erythematosus-like changes. (H&E, 100×). B – Dermoscopic features of the skin lesions. Irregular sharply demarcated erythematous lesion with erosion covered with serum crust and thin scale indicating the superficial character of epidermal detachment and desquamation
Prior to the diagnosis, the skin lesions had been subjected to treatment with topical antibacterial and antifungal medications, but no improvement was observed. The application of topical ointments containing 0.03% tacrolimus and mometasone furoate resulted in minimal improvement. Skin lesions on the face were persistent. Following the confirmation of a diagnosis of pemphigus erythematosus, a treatment plan was initiated with the administration of hydroxychloroquine at a dosage of 200 mg orally once daily. This therapeutic approach was adopted with the aim of managing the symptoms associated with the disease. Over the course of the subsequent 2 months, the cutaneous lesions exhibited a gradual resolution, resulting in the presence of only modest scaling accompanied by minor underlying erythema, as depicted in Figure 1 B.
The description of lupus erythematosus and pemhigus foliaceus features are depicted in Table 1. In summary, pemphigus erythematosus is a rare entity, but in children population it is exceptional. Although immunological confirmation is not available in our case, dermatoscopic and histopathological images as well as a good response to hydroxychloroquine treatment validate the Senear-Usher syndrome diagnosis. Pemphigus erythematosus is typically confined to the face prominence and has a good prognosis; however, facial erosions are often disfiguring and may not respond to topical therapies only. There is a paucity of randomized controlled trials and standardized treatment guidelines for children; most of the recommendations are based on case reports; therefore, the evidence regarding optimal therapy is limited. In general, corticosteroids, often in combination with other immunosuppressants such as azathioprine, mycophenolate mofetil, cyclophosphamide, and methotrexate [9–11], represent the first-line treatment for all types of pemphigus. Hymes et al. [12] reported hydroxychloroquine to be efficacious in the pemphigus foliaceus treatment of their 3 patients. Lyde et al. [13] reported a similar case of a 5-year-old girl who developed blistering skin eruptions 2 days after a dental procedure. Initially appearing on her face, the blisters soon spread to her trunk and extremities. Despite courses of oral antibiotics and topical steroids providing minimal improvement, the condition persisted. Based on clinical and immunohistological criteria, the diagnosis of pemphigus erythematosus was established. Treatment involved oral prednisone (1 mg/kg/day) and dapsone (50 mg/day). Over the next 2 months, the cutaneous lesions gradually resolved, leaving only malar scaling with minimal underlying erythema. The prednisone dosage was slowly reduced while the dapsone dosage increased to 75 mg/day. Throughout the 2-year follow-up period, there were no signs or symptoms of systemic lupus erythematosus.








