Introduction
The European Academy of Allergy and Clinical Immunology (EAACI) reported in 2014 that 6–17% of Europeans were affected by food allergy [1]. In 60% of the food-allergic adults and children, food allergy co-exists with inhalation allergy [2]. This is due to two phenomena, namely co-sensitisation and cross-reactivity. Co-sensitisation is defined as the presence of allergen-specific IgE (asIgE) for both inhalant and food allergens in a patient. Cross-reactivity, on the other hand, refers to a situation where an IgE antibody originally produced in response to one allergen binds with a similar allergen but of a different origin [3]. Cross-reactions most commonly occur between pollen allergens and foods of plant origin belonging to the same family of proteins and are caused by ubiquitous and structurally similar panallergens [4]. Panallergens contain highly conserved regions of amino acid sequences and share a similar three-dimensional structure, thus meeting the requirement for mutual cross-recognition by IgE [3–6].
Lipid transfer proteins (LTP) belong to the prolamin superfamily of proteins, are ubiquitous in the plant kingdom, predominate among the fruits of the Rosaceae family, and are classified as panallergens. LTPs are associated with the defence system of plants and are responsible for protecting the plant from bacterial and fungal infections, and belong to the PR-14 family of defence proteins. Plant LTPs are subdivided into specific and non-specific (nsLTP), and the latter have been demonstrated to cause allergy (Table 1) [7]. LTPs are also subdivided based on molecular mass into three types: LTP1 (9 kDa), LTP2 (7 kDa) and LTP3 (11 kDa). These proteins are resistant to external factors, such as high temperature or pepsin. LTPs are involved in transporting monomers required for the formation of cuticle on the surface of plant organs, which is why they mainly accumulate in the external tissues of plants, the skin and the peel. Concentrations of LTP in a single plant vary, depending on the plant maturity, storage conditions and species [6].
Table 1
Selected lipid transfer proteins recognised as allergens [7]
Aim
The aim of our study was to assess the incidence of specific IgE to components belonging to LTP based on molecular testing (ImmunoCAP ISAC) in adults with pollen allergy.
Material and methods
A total of 50 adults were included in the study: 30 women and 20 men, aged 18–74 years (mean: 35.8; SD: 12.8; median: 34), with a diagnosis of aerogenic allergy established on the basis of the history and positive skin prick tests, who developed adverse symptoms after consuming foods. The subjects were recruited from among patients of the Department of Allergy, Clinical Immunology and Internal Diseases, Ludwik Rydygier Collegium Medicum in Bydgoszcz, Poland, and the Allergy Outpatient Services of University Hospital No. 2 in Bydgoszcz, Poland, between 1.06.2014 and 1.06.2017. Severe chronic medical conditions, autoimmune diseases, cancer, allergen-specific immunotherapy, and intake of medications that could interfere with the study results were the main exclusion criteria. Patients below 18 years of age, pregnant women, and breastfeeding women were also excluded from the study,
Each subject had a targeted medical history taken, underwent a physical examination, and had their asIgE levels determined with ImmunoCap ISAC. Each subject had fasting venous blood collected from a cubital vein with a Vacutainer closed system into a tube without an anticoagulant. The blood samples were centrifuged at 3000 revolutions per minute for 15 min at room temperature using a Sigma 2K15 centrifuge, and the resulting serum was aliquoted into Eppendorf tubes and stored at –20°C for further analysis. ISAC is a test for semi-quantitative determination of IgE in serum samples. The solid phase in this test is provided by the surface of a plate on which 112 components (43 native and 69 recombinant) have been adsorbed and arranged in triplets. The ImmunoCAP ISAC test was performed according to the manufacturer’s instructions. Prior to the testing, the stored serum of a subject was thawed. Using a pipette, 20 µl of a subject’s serum was applied on one of the four testing fields available on the chip and incubated at room temperature for 120 min in the wet chamber provided by the manufacturer. After incubation, the chip with serum was washed with a washing solution for 10 min, then washed with demineralised water for 5 min, and dried. In the subsequent step of the analysis, 20 µl of a solution containing the so-called fluorescence labelled secondary antibodies to human IgE were applied with a pipette on the testing field of the chip. The plates were then incubated at room temperature for 60 min in the wet chamber. The final step of preparing the chip for reading involved washing it with Solution A for 10 min and with demineralised water for 5 min, followed by complete drying. The surface-bound allergen components react with specific antibodies present in the serum sample. The specific IgE antibodies bound to the support surface are demonstrated by adding fluorescent labelled antihuman IgE antibodies. Fluorescence was quantified using a LuxScan 10K/A scanner from CapitalBiO. The scanner was calibrated according to ISAC test manufacturer’s procedures. Scanner parameters were defined in the testing plate measurement procedure. The level of specific IgE antibodies is proportional to the measured intensity of fluorescence. The result was processed on a computer using Microarray Image Analysis (MIA) software. Antibody levels were expressed in standardised units, ISU-E (ISAC Standardised Unit for specific IgE). The measured values ranged from 0.3 to 100 ISU-E, and values ≥ 0.30 ISU-E were considered to be positive results.
Participation in the study was voluntary. The research topic and the methodology were approved by the Bioethics Committee at the Nicolaus Copernicus University in Torun, Collegium Medicum in Bydgoszcz (approval No. KB 323/2014).
Results
ImmunoCAP ISAC detects specific antibodies to the following LTP components: Pru p 3, Cor a 8, Jug r 3, Ara h 9, Tri a 14, Pla a 3, Ole e 7, Par j 2, and Art v 3. Specific antibodies to LTP were detected in 12 (24%) subjects (range: 0.3–34 ISU-E; mean: 5.26 ISU-E). The frequency, mean, standard deviation, median, minimum value, and maximum value were calculated for the detected levels of specific IgE belonging to LTP. The data are presented in Table 2.
Table 2
Descriptive statistics for levels of specific IgE belonging to LTPs
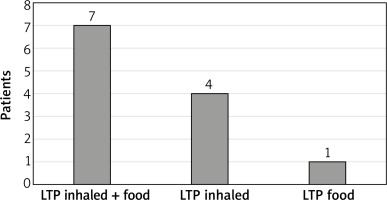
On average, subject with detectable asIgE to LTP had asIgE for 3.5 LTP components belonging. However, in the largest number of subjects, 5 (41%), antibodies to just one LTP component were detected (4 subjects with isolated allergy to Art v 3, 1 subject with isolated allergy to Pru p 3). Seven subjects had allergy to LTP components that were inhalant and food allergens, 4 subjects to inhalant LTP only, and 1 subject to food LTP only. The data are given in Figure 1.
In 11 out of 12 subjects allergic to LTP, ISAC demonstrated simultaneous presence of asIgE to components of other panallergens. Antigen-specific IgE to PR10 proteins (Bet v 1 homologues) were detected in 9 (75%) subjects, asIgE to storage proteins (SP) in seeds were detected in 3 (25%) subjects, asIgE to profilin in 2 (16.6%) subjects, asIgE to tropomyosin in 1 (8.3%) subject, and asIgE to serum albumin in 1 (8.3%) subject.
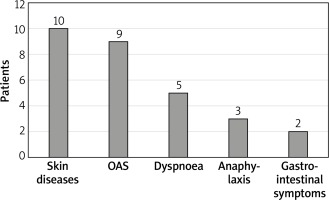
The ISAC test results were analysed statistically and clinically with details from the subjects’ histories. The largest number of subjects with detected asIgE to LTP components, namely 10 subjects, reported adverse skin reactions (urticaria, angioedema, atopic dermatitis). Anaphylaxis occurred in 25% of subjects allergic to LTP. More than one adverse symptom (mean: 2.92) following ingestion of a sensitising food were reported by 11 out of 12 (91.1%) subjects. None of the subjects presented with isolated symptoms of oral allergy syndrome (OAS). Only one of the subjects with LTP allergy reported an isolated episode of anaphylaxis. Detailed data are provided in Figure 2.
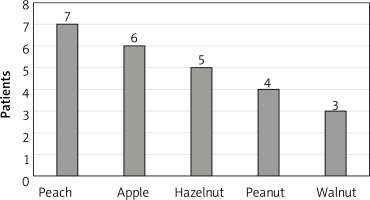
Analysis of the ISAC test results in the context of the subjects’ histories revealed that adverse reactions most commonly occurred after consumption of peaches (7 subjects). Detailed data on sensitising foods are provided in Figure 3.
Discussion
LTPs are the most common cause of food-induced allergy in adults inhabiting the Mediterranean region [8]. This is attributed to the fact that the local diets are rich in fruits of the Rosaceae family (peach, apricot, quince, almond). The first LTP fully identified and characterised as an allergen was a major allergen of peach, Pru p 3. Peach is the most common cause of LTP-associated allergy, and Pru p 3 is also considered to be a precursor of allergy to other nsLTPs [9–11].
The incidence of LTP-associated food allergy varies. A study by Nucera et al. from Italy [12] demonstrated that the incidence of LTP allergy in subjects with food allergy was as high as 84.1%. A different incidence of LTP allergy was reported by Faber et al. [13] from Belgium, who demonstrated antibodies to LTP in 24.6% of the subjects. These results are consistent with those of our study, in which asIgE to LTP were detected in the serum of 24 subjects. The differences in the incidence of LTP allergy are most likely due to the geographical region. The incidence of LTP allergy is higher in Italy than in Poland or Belgium and this difference is due to the differences in diets (different amount of fruit of the Rosaceae family consumed in both regions).
Clinically, LTP allergy manifests with severe anaphylactic reactions or milder reactions, such as oral allergy syndrome (OAS) [9]. In subjects with detected asIgE to LTP components, the largest number of patients reported cutaneous symptoms (83%), followed by OAS (75%), shortness of breath (41.5%), and gastrointestinal symptoms (16.6%). Anaphylactic reaction occurred in 25% of subjects allergic to LTP. Our results are for the most part consistent with findings reported by Pascal et al. [14]. In their study, allergy to LTP manifested with: OAS in 75.6% of the subjects, urticaria in 66.7%, gastrointestinal symptoms in 55.6%, and anaphylaxis in 75.6%. The higher rate of anaphylaxis in the study by Pascal et al. may be due to the differences in eating habits within the study population. The study was conducted in Barcelona, Spain, where consumption of fruits of the Rosaceae family is much higher than that in Poland.
Peach is the most common cause of LTP-associated allergy. In our study, subjects allergic to LTP most commonly reported adverse reactions after consuming a peach, an apple or nuts. In a study by Aseero [15], five foods that most commonly caused symptoms in patients with LTP were identical to those in our study. In Aseero study, the most common sensitising foods were peach (100%), walnut (60%), apple (54%), hazelnut (46.6%), and peanuts (40%).
The major peach allergen Pru p 3 is also considered to be a precursor of allergy to other LTP. In our study, asIgE for Pru p 3 was demonstrated in the serum of 6 subjects. The most common sensitising LTP was Art v 3 (an Artemisia vulgaris protein), with asIgE demonstrated in 9 subjects. Furthermore, asIgE to Jug r 3 and Pla a 3 were detected in 6 subjects, to Ara h 9 in 5 subjects, to Cor a 8 in 4 subjects, to Ole e 7 in 3 subjects, to Tri a 14 in 2 subjects, and to Par j 2 in 1 subject. The highest mean levels of asIgE were identified for: Art v 3 (8.36), Ara h 9 (6.16), Pru p 3 (5.45), Cor a 8 (5.28), Jug r 3 (4.8), Pla a 3 (3.62), Tri a 14 (3.15), Par j 2 (1.5), and Ole e 7 (0.93). Our results differ from those of Pascal et al. [14]. In the latter study, the incidence rates of allergy to LTP components were as follows: 100% for Pru p 3, 68.9% for Pla a 2, 64.4% for Art v 3, 57.8% for Cor a 8, and 28.6% for Par j 2. The highest median ISU-E was observed for: Pru p 3 (3), Pla a 2 (0.8), Art v 3 (0.6), Cor a 8 (0.5), and Par j 2 (0.3). Similar results for LTP allergy to those in our study were reported by Gao et al. [16]. The study was conducted in China, where peaches are not a very common food but the concentration of Artemisia vulgaris pollen is high. In this study, asIgE antibodies to Art v 3 were detected in 60% of the subjects, and those to Pru p 3 in 51%. The mean levels of antibodies to Art v 3 were significantly higher than those for Pru p 3.
Given the higher incidence of allergy to Art v3 compared to allergy to Pru p 3, and due to the higher mean level of ISU-E for Art v 3 compared to Pru p 3, a detailed analysis of subjects allergic to LTP was carried out. Only in 1 out of 12 subjects, no asIgE to either Art v 3 or Pru p 3 was detected. In 2 subjects, asIgE to Art v 3 and Pru p 3 was identified, but these findings were excluded from further analysis because the detected concentrations were at the limit of detection for ImmunoCAP ISAC. The remaining subjects could be divided into two groups. The first group consisted of subjects with asIgE to Art v 3 but not to Pru p 3 (5 subjects) and subjects in whom the levels of asIgE to Art v 3 were higher than those of asIgE to Pru p 3 (1 subject), giving a total of 6 out of 9 subjects (66%). The second group consisted of subjects with asIgE to Pru p 3 but not to Art v 3 (1 subject) and subjects in whom the levels of asIgE to Pru p 3 were higher than those of asIgE to Art v 3 (2 subjects), giving a total of 3 out of 9 subjects (33%). Interestingly, all the subjects with allergy predominantly to Pru p 3 and none of the subjects with allergy predominantly to Art v 3 developed anaphylaxis. Also Gao et al. [16] divided their study population into two groups, one in which allergy to Art v 3 predominated (majority of the subjects) and the other in which allergy to Pru p 3 was more prevalent.
The results of our study and the cited papers suggest a possibility of two pathways of sensitisation to LTP. The first pathway is observed in the countries of the Mediterranean region, where Pru p 3, a protein of the peach, an important dietary ingredient in this region, is the cause and precursor of LTP allergy. On the other hand, in countries where peaches are not an important ingredient of the diet, but concentrations of Artemisia vulgaris pollen are high, Art v 3, a component of Artemisia vulgaris pollen, is the cause and precursor of LTP allergy. Also Garcia-Sellies et al. [17] and Lombardero et al. [18] address the two possible mechanisms of allergy to LTP in their papers.
Conclusions
Specific IgE antibodies to LTP components were identified in 24% of the subjects in our study population. In subjects with LTP allergy, adverse skin reactions were the most common after consumption of sensitising foods. Peach was the most common food allergen triggering these reactions. The Artemisia vulgaris component Art v 3 was the precursor of LTP allergy in our study population: the highest incidence and the highest mean levels of asIgE were demonstrated for this component.