Introduction
Atopic dermatitis (AD) is a common, chronic inflammatory skin condition with severe and persistent pruritus as its diagnostic hallmark. Recurrent course of the disease and its troublesome symptoms often lead to serious socio-economic consequences. Moreover, in severe cases, AD might constitute a significant therapeutic challenge.
Pathogenesis of AD is a resultant of hereditary, immunological and environmental factors, which all contribute to the skin barrier defect. Currently, the immunology of AD and its curability with targeted therapies has become a major focus of attention of the scientific societies worldwide.
Interleukin 33 (IL-33) is an inflammatory cytokine considered important in the pathogenesis of AD. IL-33 is known to promote T helper type 2 (Th2) and mast cell responses through the suppression of tumorigenicity 2 (ST2) and IL-1 receptor accessory protein receptors IL-1RAcP [1]. Consequently, IL-33 may influence Th2-related diseases such as asthma, AD and allergic rhinitis. Furthermore, IL-33 belongs to the alarmin family, as it is released by necrotic cells after tissue damage [2, 3]. Biological activity of IL-33 is independent of caspase-1 cleavage [4]. Accordingly, IL-33 can be released inside the injured skin while scratching.
Previous studies revealed an increased expression of IL-33 mRNA in keratinocytes and endothelial cells of patients with AD [5]. Its impact on the skin barrier has also been investigated. IL-33 has a potency to downregulate filaggrin expression in human keratinocytes [6]. Moreover, it is considered to play an essential role in clinical allergic and anaphylactic responses [7].
Aim
The present study constitutes an attempt to determine the way in which polymorphisms in the 9894 T/C (rs1929992) and 11877 C/T (rs10975519) loci of the IL-33 gene and the serum concentration of IL-33 correlate with the severity of the disease, intensity of pruritus, age of onset, serum IgE levels, age, sex, occurrence of the disease, sleep disturbances and concomitance of asthma in the population of patients with AD from Northern Poland. To our knowledge, this is the first study of that kind. The study was conducted in 2012-2019, however we find it particularly significant in 2021 in the light of ongoing studies on etokimab and in the beginning of the era of biologic treatment in AD [8]. We believe that results of this research stay in line with new concepts of personalized medicine, stress the significance of AD immunotype, genotype and ethnic differences in this field and could be helpful to define the group of patients, who would benefit most from the biological therapy.
Material and methods
This retrospective, cross-sectional study included 191 patients with AD and 168 healthy controls. The study group consisted of patients from the Department of Dermatology and Dermatological Outpatient Clinic of the University Clinical Centre in Gdansk in 2012–2019. Patients with autoimmune diseases, malignancies or undergoing systemic treatment or immunotherapy were excluded from the research. The control group consisted of healthy volunteers. The groups were representative for the population of Northern Poland. The clinical characteristics of the patients and the members of the control group are summarized in Table 1. The samples did not vary significantly regarding age and sex.
Table 1
The comparison of age of the patients and the members of the control group. The table shows the analysis of age of patients with atopic dermatitis and members of the control group. The mean age, standard deviation (SD), median age, median minimum age (Q25) and median maximum age (Q75)
The diagnosis of AD in each patient was established after collecting medical history and performing a thorough dermatological examination using the Hanifin and Rajka criteria [9]. The patients’ blood samples were drawn with the help of VACUETTE® blood collection equipment, according to the WHO phlebotomy guidelines. Each participant’s blood specimens were collected into two types of tubes: 2 ml into the tube containing 8% potassium edetate (EDTA-K3) and 4 ml into the tube containing a clot activator. The latter tubes underwent the centrifugation process and the obtained blood serum samples, along with the EDTA-K3 tubes, were stored in the temperature of –80°C until the laboratory testing. DNA samples were isolated from peripheral blood with the Blood Mini DNA extraction kit, according to the manufacturer’s instructions (A&A Biotechnology, Gdañsk, Poland). The serum level of IL-33 was estimated with the help of ELISA kit. Single nucleotide polymorphisms (SNPs) in the 9894 T/C (rs1929992) and 11877 C/T (rs10975519) loci of the IL-33 gene were assessed by the amplification refractory mutation system - polymerase chain reaction (ARMS-PCR) method. Total IgE levels were measured using fluoroimmunoenzyme assay (UniCAP 100; Phadia, Uppsala, Sweden), following the manufacturer’s instructions. The cut-off point for serum IgE was 100 kU/l. The assessment of pruritus severity was performed with the Visual Analog Scale (VAS) in which 0–3 pts stand for mild, 3–7 moderate, 7–9 severe and > 9 pts very severe intensity of pruritus [10]. Severity of AD was assessed with the SCORAD (Scoring Atopic Dermatitis) index, the result of < 25 pts was classified as mild, 25–50 pts moderate, whereas > 50 pts as severe [11].
The concentration of IL-33 and its genetic variants has been compared with the concentration of IgE, severity of pruritus and severity of AD in participants of the study.
The study was assessed and approved by the Ethics Committee. Written consent was obtained from all patients prior to enrolment in the study.
Statistical analysis
The χ2 analysis was employed to evaluate the significance of differences in the observed allele between groups. A logistic regression model was used to calculate the odds ratios (ORs) and the 95% confidence intervals (CIs). The Mann-Whitney U-test and Kruskal-Wallis test were used to compare the median values. Analyses were performed using the Statistica 12.0 software package (StatSoft, Inc.). The value of p < 0.05 was considered statistically significant.
Results
The results did not reveal any differences in serum levels of IL-33 between patients with AD and healthy controls (p = 0.827) (Figure 1).
Figure 1
Comparison between serum level of IL-33 in patients with atopic dermatitis and in healthy controls
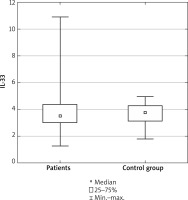
The IL-33 level was not associated with sex, age of patients, age of onset, IgE level (p = 0.063), SCORAD (p = 0.059), pruritus (p = 0.15) or other atopic conditions.
The study showed no statistical correlation regarding the IL-33 gene polymorphisms (11877, p = 0.44; 9894, p = 0.97) differences between the study and the control group (Table 2). As regards the 11877 locus, the CC genotype appeared to be the most frequent, whereas TT was the least common, however it was not statistically significant. Similarly, the study of frequency of C and T allele in the 9894 locus showed that TC dominated both in patients with AD and in the control group.
Table 2
Interleukin 33 genotypes and allele frequencies. The table describes frequencies of patients alleles of IL33-11877 and IL33-9894 both in patients with atopic dermatitis and the study group. P-value is also determined
The research revealed no statistically significant correlation between the concentration of IL-33 in the blood serum and SCORAD (p = 0.059), or between specific IL-33 polymorphisms and the severity of the AD (11877, p = 0.94; 9894, p = 0.69).
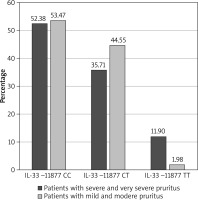
The participants’ level of pruritus was classified as very severe, severe, moderate or mild. There were no correlations between the serum concentration of IL-33 and pruritus (p = 0.15). According to the results, in case of the IL-33 SNPs in locus 11877, patients most often reported mild or moderate pruritus. The TT genotype in the 11877 locus was more frequently observed in patients with severe and very severe pruritus (p = 0.01): TT genotype was present in 11.90% of patients with severe and very severe pruritus versus 1.98% of patients with mild and moderate pruritus (Figure 2). The TT genotype increased the risk of occurrence of pruritus: OR = 6.69 (95% CI: 1.24–35.99), p = 0.03. No significant correlation was observed between the 9894 polymorphism and the severity of pruritus.
The study demonstrated no significant correlations between IgE concentration and IL-33 levels (p = 0.15) or between the concentration of IgE and IL-33 gene polymorphisms (11877, p = 0.54; 9894, p = 0.41) (Table 3). No differences of genotypes were found between the patients with AD and the patients with asthma.
Table 3
Comparison of immunoglobulin E (IgE) concentrations for individual genotypes [kU/l]. The table shows the concentration of IgE in particular genotypes. The mean value and the median value were the highest in patients with the TT genotype
There had been no correlation between VAS score and concentration of IL-33 as well.
We found no correlation between age, age of onset, sex, serum levels of IL-33 or SNP.
Discussion
Atopic dermatitis is a chronic inflammatory disease resulting from both genetic and environmental factors, characterized by production of the Th2 cytokines including i.e. IL-4, IL-5 and IL-13. IL-33 is thought to be a potent initiator of the Th2 immune response. As IL-33 binds and activates its receptors in mast cells, dendritic cells, basophils, eosinophils, innate lymphoid cells and Th2 cells, it causes the release of the cytokines and exacerbation of the allergic inflammation. However, we found no differences between IL-33 levels in AD patients and healthy volunteers. A previous study compared the level of IL-33 with demographic features of the population of patients with AD. It has been proven that the IL-33 level is not affected by family history, sex or age of patients. It was also stated that the duration of AD may contribute to a higher level of IL-33 in the blood serum. Nevertheless, there was no correlation between the IL-33 serum level and association with other atopies [12]. These results are consistent with the ones from this study.
Polymorphisms in the IL-33 and interleukin-1-receptor-like-1 (IL1RL1) genes can act as either protective or risk factors for asthma or allergy in humans. However, a small number of studies have been published so far to replicate such findings in the European population of patients with AD.
According to the literature, chitin, a structural component of fungi and other primitive organisms, may increase the IL-33 concentration in the lungs, which triggers IL-1β production by the dendritic cells (DCs) [13]. The expression of the IL-33 receptor on the basophil membrane was at baseline significantly higher in patients with severe asthma in comparison to those with a mild or moderate course of the disease and in healthy subjects as well. The level of IL-33 receptors increased after IgE stimulation [14]. We detected no correlation between the IL-33 concentration and IgE level. Nevertheless, our group consisted solely of AD patients or patients with AD and concomitant asthma, which may have potentially affected the results.
We hypothesized that patients with AD may experience different course of the disease depending on the diversified distribution of allele frequencies. Especially having regard to the fact that genetic variants of IL-33 may have a strong effect on its biological activity.
Selection of polymorphisms of IL-33 that constitute the main focus of this research was based on the results published by Sakashita et al. in 2008. Authors of this study analysed the HapMap database and determined 22 single nucleotide polymorphisms of the IL-33 gene and investigated the association of these polymorphisms with Japanese cedar (JC) pollinosis, the most common form of allergic rhinitis in Japan, the disease which, alike AD, is associated with an impaired Inflammatory Response Pathway. Based on statistical analysis, a significant relationship between SNP in the 9894 T/C locus (rs1929992) of the IL-33 gene and the occurrence of Japanese rhinitis has been demonstrated (p = 0.048) [15].
Other study determined relations between polymorphisms mentioned above and the course of mycosis fungoides (MF). MF is the most common form of cutaneous T-cell lymphoma, and similarly to AD, patients suffering from MF seek medical help because of severe pruritus. This study evaluated whether particular polymorphisms have a prognostic significance for the disease or whether they might affect the symptoms of AD. No such correlation has been found. Nevertheless, similarly to our results, patients with the TT genotype in the 11877 locus of the IL-33 gene had higher scores in the Visual Analogue Scale (VAS) which measured the intensity of pruritus [16].
The knowledge about impact of respective polymorphisms of the IL-33 gene is constantly progressing. There have been reports that showed the correlation between specific genotypes of this cytokine and higher susceptibility to rheumatoid arthritis [17]. AD is also considered a disease that reveals a rising tendency to coexist with other autoimmune disorders [18]. In our group, no patient suffered from AD and concomitant rheumatoid arthritis. Nevertheless, the association of these diseases might set a new research focus worldwide. The same applies to a widely discussed association of AD and cardiovascular diseases [19], IL-33 is known for its protective role in atherosclerosis [20]. Increased cardiovascular and atheromatous markers were detected in blood of older patients with AD [21]. It was demonstrated that the same factors are involved in AD and pathogenesis of atherosclerosis, moreover the role of IL1RL1 appeared to be also important in this process [22, 23]. Our study group included very few patients with these comorbidities not allowing to find any reliable results, but we plan further studies in this field.
Conclusions
Numerous studies have been performed in order to explain the genetic background of AD. Recent findings suggest that variation in immune-mediated pathways not only plays a key role in AD development, but it is also substantial for intact skin barrier function. Mutations of the filaggrin encoding gene are thought to be most significant risk factor for development of AD. Nevertheless, only a minor part of genetic factors contributing to the cause of AD can be currently elucidated. Moreover, large multi-centre studies comprehensively combining gene-gene and gene-environment interactions and epigenetic mechanisms may further explain the genetic factors underlying AD pathogenesis and subsequently allow more individualized treatment in the future.
Undoubtedly, personalized therapy should always be considered in the shed of genetic and immunologic endotypes, regarding the patient’s background. In our study, we proved that specific genetic variants of IL-33 might influence the clinical course of the disease, especially regarding the symptoms. Furthermore, we demonstrated that the particular genetic polymorphisms of IL-33 and its serum concentration have no correlation with the level of IgE antibodies, neither with the severity of the disease.
Our study proves that particular interleukins in AD might impact specific symptoms of the disease.
It also shows that AD is a very heterogeneous disease and its course differs in individuals. This is the first study that distinguishes a group of patients who benefit most from the prospective therapy with anti-IL33 treatment.
The main restriction of this research is the limited number of members of the study group and diversified number of patients with particular polymorphisms. Large-scale studies are needed to reveal the relationship between the IL-33 serum concentration and its gene polymorphism and the clinical course of AD.