Introduction
Psoriasis is a multisystem, chronic inflammatory skin disease, which affects about 1–3% of the Caucasian population. It is immune-mediated disease in which T-lymphocytes and dendritic cells play a pivotal role. Clinically psoriasis is manifested by hyperproliferation and abnormal differentiation of the epidermis, inflammatory cell infiltration and vasodilation. Many recent reports have indicated that psoriasis is associated with multiple systemic disorders such as obesity [1, 2]. The development of both diseases, which are non-infectious, depends on the genetic predisposition, lifestyle and environmental factors [3].
It is generally known that obesity is a major risk factor for the metabolic syndrome which includes abdominal obesity, insulin resistance, hypertension and atherogenic dyslipidemia [3]. The pathogenesis of psoriasis and obesity is closely related due to the increased release of proinflammatory cytokines and lipid profile disturbances. Numerous studies have shown that psoriasis is associated with an increased prevalence of metabolic syndrome [4]. On the other hand, it is also believed that obesity is an independent factor in the development of psoriasis and its presence deteriorates prognosis [4–7].
Ceramides (CERs), next to cholesterol and free fatty acids, are epidermal lipids that play a key role in the skin barrier function [8, 9]. Sphingosine-1-phosphate (S1P) belongs to a group of sphingolipids and recently they constituted the object of interest of many research studies [10]. Reduction in sphingolipids is connected with a transepidermal water loss and an epidermal barrier dysfunction, which may lead to the inflammatory skin diseases [11]. It is proven that ceramides, being a component of stratum corneum, are responsible for various processes such as proliferation, differentiation and apoptosis [12]. Moreover, CERs participate in the cell cycle arrest, inflammatory and stress responses [13]. In many dermatological diseases, including psoriasis, the concentration of ceramides in the skin and their circulating levels in serum differ from these in healthy people. Reduced CER levels in psoriatic skin are associated with their anti-apoptotic effect with concomitant pro-proliferative action, which explains the occurrence of psoriatic papules [14]. In our recent study we have also shown a decrease in circulating CERs in psoriatic patients compared to healthy subjects [13].
Ceramides take part in metabolic disturbances and thus they contribute to the development of such diseases as type 2 diabetes, cardiovascular disease, atherosclerosis and obesity [15, 16]. For example palmitic ceramide (C16:0) is recently considered as the principal mediator of obesity-derived insulin resistance, impaired fatty acid oxidation and hepatic steatosis. As it is commonly known, the reduction in fatty acid oxidation is observed in obesity, which can cause lipid accumulation within the cells, conferring more susceptibility to cell dysfunction [17]. Sphingosine-1-phosphate (S1P) is a bioactive molecule which induces a wide spectrum of cellular effects, including proliferation, differentiation, survival and migration. It can also take part in the pathogenesis of several diseases such as cancer, myocardial infarction, autoimmunity, osteoporosis and atherosclerosis [18–21].
There are limited data on the relation of CER disturbances in psoriatic patients with obesity.
Aim
The aim of our study was to evaluate selected circulating CER and S1P levels in psoriasis in relation to the concomitant weight disturbances and their association with the course of psoriasis and selected inflammatory and metabolic markers.
Material and methods
Patients
Eighty-five patients were included in the study, among which twenty eight individuals were women and fifty seven were men. An active plaque-type psoriasis was confirmed in all cases. The age of patients ranged from 19 to 79 years (median ± 53). The control group consisted of 32 healthy persons, which were sex- and age-matched. Severity of psoriasis was estimated using the Psoriasis Area and Severity Index (PASI). Other types of psoriasis (such as pustular psoriasis, erythrodermic psoriasis, psoriatic arthritis) were excluded from the study.
All examined patients were deprived of any systemic drugs for a minimum 1 month before the study. According to body mass index (BMI; kg/m2), all patients were divided into three groups: (1) with normal body weight (BMI < 25 kg/m2), (2) overweight (25 kg/m2 ≤ BMI < 30 kg/m2) and (3) obese patients (BMI ≥ 30 kg/m2). None of the patients and control individuals was covered by any diet restriction. Clinical data and laboratory results were collected from medical records of patients hospitalized at the Dermatology and Venereology Department of the Medical University in Bialystok. All biochemical analyses were carried out by the Central Laboratory of the University Hospital Centre.
All patients and controls gave their written informed consent before the enrolment. The study was approved by the local bioethical committee.
Biochemical analysis
Peripheral blood samples, after overnight fasting, had been taken from patients and control group before the treatment started. After centrifugation, the serum was collected and stored at –80ºC until further analyses were performed. There are many different sphingolipid subfractions and their related isoforms, and thus we have chosen the most biologically active forms. We have evaluated the serum concentration of ceramide and its 14 different species (C14:0, C16:0, C16:1, C18:0; C18:1; C18:2; C20:0; C18:3; C22:0; C20:4; C24:0; C20:5; C24:1; C22:6) as well as sphingosine-1-phosphate.
The serum samples were mixed with a solution composed of 25 mM HCl and 1M NaCl and acidified with methanol. Internal standards of C17-sphingosine and C17-sphingosine 1-phosphate (Avanti Polar Lipids, Alabaster, AL, USA) were added. Lipids were extracted by means of chloroform, 1 M NaCl and 3N-NaOH. The aqueous phase that contained S1P was moved to a fresh tube and the compound was dephosphorylated with the use of alkaline phosphatase (bovine intestinal mucosa, Fluka). Free sphingosines were converted to their O-phthalaldehyde derivatives and analysed by means of high performance liquid chromatography (HPLC) system equipped with the fluorescence detector and C18 reversed-phase column (Varian Inc., OmniSpher 5, 4.6 × 150 mm).
In order to quantify CER, a small volume of the chloroform phase containing lipids was transferred to a tube containing N-palmitoyl-D-erythro-sphingosine (C17 base) as an internal standard. The lipid fractions were separated by thin-layer chromatography silica plates (Kieselgel 60, 0.22 mm, Merck, Darmstadt, Germany) with a heptane : isopropyl ether : acetic acid (60 : 40 : 3, v/v/v) resolving solution was used. Lipid bands were visualized by spraying with a 0.2% solution of 3’7’-dichlorofluorescin in methanol and identified under ultraviolet light using standards on the plates. The gel bands were scraped off the plate, transferred into screw tubes and transmethylated with BF3/methanol. The fatty acid methyl esters (FAMEs) were resolved in hexane and analysed by gas-liquid chromatography. A Hewlett-Packard 5890 Series II gas chromatograph with Varian CP-SIL capillary column (50 m 0.25 mm internal diameter) and flame-ionization detector (Agilent Technologies, Santa Clara, CA) were used. Injector and detector temperatures were set at 250°C. The oven temperature was increased linearly from 160°C to 225°C at a rate of 5°C/min. According to the retention times of standards, the individual long-chain fatty acids were quantified. Total content of CER was evaluated as the sum of the particular fatty acid species of the assessed fraction and it was expressed in nanomoles per millilitre of the serum.
Statistical analysis
Data were presented as median and quartiles (first and third quartile) and percentage, when appropriate. After evaluation of distribution, statistical analysis was carried out using Kruskal-Wallis and Mann-Whitney tests. Results were considered to be statistically significant at p< 0.05. Moreover, correlations between the variables were calculated using non-parametric Spearman’s test.
Results
Eighty-five patients with exacerbated plaque-type psoriasis and 32 healthy controls were included in the study. The mean age was 49.7 ±14.4, which ranged from 19 to 79 years. The average duration of psoriasis was 18.5 ±14.4 months (from 1 to 58 months). Mean PASI score was 11.4 ±8.7. In the examined group, 50 (58.8%) patients had a mild (PASI < 10), 22 (25.8%) moderate (10 < PASI < 20) and 13 a severe form of psoriasis (PASI > 20). The mean BMI of all examined subjects was 28.5 ±6.3. Most of them (n = 30) were overweight patients (35%), 27 (32%) suffered from obesity and 28 (33%) persons had normal weight. Selected demographic, clinical and laboratory patient data are presented in Table 1.
Table 1
Clinical characteristics of the examined group of psoriatic patients (n = 85)
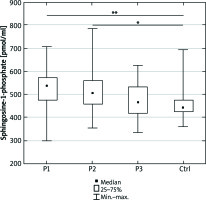
We did not observe any significant differences in the total serum ceramide concentration between the examined and control group (Figure 1). S1P concentration was higher in psoriatic patients with normal body weight as well as overweight compared to the control group (p = 0.002 and p = 0.04, respectively) (Figure 2).
Figure 1
Total ceramide concentration in the serum of the psoriatic patients with normal body weight (P1), overweight (P2), obesity (P3) and the control group (Ctrl). Data shown as median (Q1, Q3)
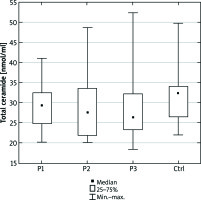
Figure 2
Sphingosine-1-phosphate concentration in the serum of the psoriatic patients with normal body weight (P1), overweight (P2), obesity (P3) and the control group (Ctrl). Data shown as median (Q1, Q3). Significant differences in the control group are shown as: p < 0.05* and p = 0.01**
We have also shown disturbances of selected CER concentration in particular groups of patients in comparison to the healthy controls. These results are shown in Table 2.
Table 2
Differences between serum ceramides (C) (nmol/ml) and sphingosine-1-phosphate (S1P) (pmol/ml) concentrations in psoriatic patients with different body weight. Data are shown as median and quartiles (Q1, Q3), (*min.–max. value)
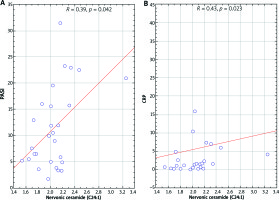
In psoriatic patients with normal body weight, nervonic ceramide (C24:1) correlated with PASI (r = 0.38; p = 0.042) and C-reactive protein (CRP) (r = 0.42; p = 0.023). Correlations are shown in Figure 3. We have also observed a negative correlation of eicosapentaenoic ceramide (C20:5) with PASI (r = –0.47; p = 0.022) in this group of patients.
Figure 3
Scatterplot of nervonic ceramide (C24:1) correlation with PASI (A) and C-reactive protein (B) in psoriatic normal weight patients
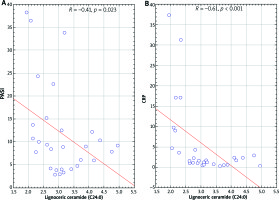
In overweight patients, the concentration of lignoceric ceramide (C24:0) correlated inversely with the severity of the disease (r = –0.41; p = 0.022), CRP (r = –0.6; p = 0.0004) (Figure 4) and platelet count (r = –0.49; p = 0.01). In addition, in these patients the serum concentration of palmitoleic ceramide C16:1 inversely correlated with severity of disease (r = –0.39; p = 0.03). Other ceramide concentrations did not correlate with severity of psoriasis or inflammatory markers.
Discussion
According to the current literature, CERs and other sphingolipids are associated with the development of atherosclerosis [22, 23], risk of an incidental cardiovascular event [24], non-alcoholic fatty liver disease [25] and colorectal cancer [26]. Even though the role of circulating CERs is widely studied, there are still limited data on their role in psoriasis and its comorbidities.
In the present study we have examined circulating CER concentration in psoriatic patients with respect to the body weight. We have revealed, though without any statistical significance, a lower total serum CER level in all patients with psoriasis compared with the healthy subjects. Nevertheless, we have demonstrated significant differences in the concentrations of individual CERs in obese, overweight and normal body weight patients (Table 2). CER concentration disturbances in psoriatic skin lesions were previously reported in a few studies [27, 28]. The low total serum CERs concentration in our group of patients probably reflects their low content in the psoriatic epidermis. To our knowledge, there are no studies evaluating the correlation between the ceramide content in the healthy skin or psoriatic lesional skin and the circulating level of ceramides. In the available medical literature, we have found only one publication by Checa et al. [29] where authors reported simultaneous disturbances of CER levels in the serum and psoriatic epidermis. Based on their results we can speculate that although epidermal lipids synthesis is largely independent of systemic lipids, these two compartments may be interrelated, especially in a pathologic condition like psoriasis.
The serum S1P level was higher in all psoriatic groups than in the controls. The phenomenon of elevated S1P in obesity has been previously well established. Some authors consider S1P as a marker of obesity and insulin resistance [30, 31]. According to Kowalski et al., the concentration of plasma S1P positively correlates with clinical indices of metabolic syndrome in obesity [32]. All psoriatic patients had a higher serum concentration of S1P than healthy controls. We have demonstrated the highest S1P concentration in normal weight psoriatic patients (Figure 3). Lower S1P concentration in our obese group of patients compared to normal weight patients may reflect their greater tissue uptake and can cause reduced insulin sensitivity. S1P has anti-apoptotic and anti-inflammatory action on different cell lines (such as hepatocytes, kidney podocytes) and anti-inflammatory action on macrophages. The number of macrophages is significantly increased in obesity. They can account for up to 60% of all fat tissue masses, producing pro-inflammatory molecules and they are responsible for the development of chronic inflammation in fatty tissue and further cytokine production [4]. Results of our study may explain a worse course of psoriasis in obese patients. On the other hand, according to our study we can also speculate that psoriatic patients, even with normal body weight, due to the higher level of S1P, are more likely to develop obesity and metabolic syndrome than the healthy subjects.
In our study, palmitoleic ceramide (C16:1) and lignoceric ceramide (C24:0) were inversely correlated with severity of psoriasis measured by PASI in overweight patients. Our data about lignoceric ceramide are consistent with other reports which claim that this ceramide plays an important role in maintaining the epidermal barrier [15, 33]. We have additionally observed a negative correlation of C24:0 with inflammatory markers (CRP and platelets), what may have an additional prognostic value in assessing general inflammation in this group of patients. Ceramide C24:0 plays a positive, protective role in inhibiting the development of insulin resistance and inflammation in different organs [34] and is necessary to maintain homeostasis of the liver [22, 35]. Kasumov et al. demonstrated that decreasing the C24:0 concentration leads to increased susceptibility to insulin resistance and this in turn can lead to non-alcoholic fatty liver disease (NAFLD) [22]. Thus, the revealed low concentration of C24:0 in our study may indicate the predisposition of psoriatic patients to the development of NAFLD, which was previously described in this group of patients [22]. According to our results, a decrease in ceramide C24:0 may be a useful tool for the evaluation of inflammation and metabolic disturbances in psoriasis.
In psoriatic patients with normal body weight, nervonic ceramide (C24:1) positively correlated with PASI. Our findings stay in line with Checa et al. who showed that circulating levels of ceramides: C16:0, C18:0, C20:0, C22:0 and C24:1 are statistically increased in severe psoriatic patients [29]. In our study, more than half of all patients had a mild course of the disease and only 13 suffered from severe psoriasis. Our results indicate that C24:1 may have a prognostic value in predicting the course of psoriasis and serve as a marker of inflammation, all the more that we have observed a positive correlation of C24:1 with CRP in both normal weight and obese patients. But our findings are not consistent with some other research. Ceramide C24:1 is considered to have a protective and anti-inflammatory effect. According to Fox et al., C24:1 metabolism disturbances may contribute to diabetes complications [36]. The authors observed a decreased level of C24:1 in type 1 diabetes and its increase after insulin treatment. Thus, the disturbances of C24:1 metabolism and their meaning in psoriasis needs further investigations.
In the normal weight patients, we have observed an inverse correlation between eicosapentaenoic ceramide (C20:5) and PASI. There are limited data in the current literature about the effect of C20:5 ceramide on the skin. Kendall et al. showed that eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) supplementation can influence the content of ceramides in the skin. Taking into account the increase in ceramides after EPA supplementation, authors speculate that it can contribute to regulation of the function of fibroblasts such as differentiation of the cells and apoptosis. Finally, it may improve the general condition of the skin [6, 37]. We did not observe a similar correlation between C20:5 and PASI in overweight and obese patients in our study. Lack of this correlation may depend on C20:5 level disturbances in obesity.
This study has some limitations which have to be pointed out. First, in our group of 28 psoriatic patients with normal body weight, 4 patients had slightly elevated levels of cholesterol and no one had an increased level of triglycerides. In statistical analysis this major class of lipids did not correlate with PASI or inflammatory markers. Taking into account these results, we believe that serum content of CERs is a more sensitive lipid indicator. Second, there are some conditions (smoking, systemic treatment of other diseases) that may have an influence on our results. Diabetes and arterial hypertension were diagnosed in 3 and 5 patients, respectively, of which 3 patients suffered from both diseases simultaneously. Due to the fact that it was a small group of patients, we believe that this fact did not affect the CER level disturbances.
Conclusions
We have demonstrated CER profile disturbances in psoriatic patients with normal body weight as well as in overweight and obese patients. In our study we found a positive correlation of nervonic ceramide, and negative of lignoceric ceramide with PASI and inflammatory markers. Higher S1P serum concentration in psoriatic patients may indicate their predisposition to the development of metabolic syndrome. Further research studies are needed to assess the effect of individual ceramides on the course of psoriasis. They might have an additional predictive value for the development of metabolic syndrome in patients with psoriasis.