Introduction
Liver fibrosis is a progressive condition characterized by replacement of normal liver parenchymal tissue by fibrotic tissue with or without concomitant abnormal regenerative nodules and vascular changes [1].
Determination and staging of liver fibrosis are clinically relevant for patients’ management: the choice of the appropriate method of treatment, urgency of antiviral treatment, prioritization for liver transplantation and prediction of fibrosis progression leading to liver cirrhosis. Chronic hepatitis B and C seemed to be the most frequent reasons for progressive liver disease in Europe. New therapeutic options, which became available recently, particularly for the treatment of chronic hepatitis C virus (HCV) infection, increase the need for simple and safe methods of fibrosis evaluation.
The traditional method of hepatic fibrosis evaluation was histologic examination of a liver specimen obtained through liver biopsy, which was established a long time ago as a gold standard for the diagnosis of liver diseases. However, as an invasive technique it has some limitations, which include complications such as pain, intraperitoneal or intrapleural haemorrhage, pneumothorax, gut perforation, and local or systemic bacterial infection, which may infrequently result in patient death [2]. Moreover, with readily available non-invasive methods, the large majority of patients refuse to undergo liver biopsy, and they are even more reluctant to accept a second liver biopsy, which make it impossible to evaluate fibrotic changes resulting from the disease progression or antiviral treatment efficacy [3]. Additionally, the sampling error related to the length of the hepatic specimen and intraobserver or interobserver differences may affect the accuracy of the diagnostic results [4-6].
There have been at least several non-invasive techniques developed to assess liver fibrosis. Among them, shear-wave ultrasound-based elastography (SWE) methods have been validated as a diagnostic tools for the evaluation of the stiffness of solid organs or tissues. These techniques include transient elastography (TE; FibroScan, manufacturer Echosens), point-SWE (pSWE; Acuson, manufacturer Siemens) and two-dimensional SWE (2D-SWE; Aixplorer, manufacturer Supersonic Imagine). In TE shear weaves are induced by mechanical push; thus the results can be biased by the presence of ascites, oedema, inflammation, extrahepatic cholestasis, congestion, or obesity. In pSWE and 2D-SWE shear waves are triggered by focused high-frequency acoustic beams directly within the liver, which allows some of the limitations of TE to be overcome. However, comparing these two methods, in 2D-SWE the assessed area (region of interest – ROI) is significantly larger compared to pSWE. Recently, the comb-push ultrasound-based shear wave elastography method was developed; it enables ones to assess the shear waves by ultrasound systems with low pulse repetition frequency. However, these techniques require further clinical validation (Bende et al.). Real-time two-dimensional shear wave elastography is an ultrafast ultrasound-based tool for a non-invasive, quantitative assessment of liver tissue stiffness corresponding to hepatic fibrosis, combined with two-dimensional, real time, ultrasound imaging. 2D-SWE has an additional advantage of providing simultaneously anatomic B-mode US images and elastography colour maps according to the degree of stiffness, which offers the potential of enhanced accuracy in the assessment of liver stiffness [7, 8]. In contrast to “blind” TE, 2D-SWE enables selection of the most appropriate area (ROI) for the evaluation of liver stiffness. There is a large quantity of data supporting consistency between TE and liver biopsy in patients with chronic hepatitis B virus (HBV) or HCV infection, but a more limited amount regarding 2D-SWE. Some studies provide evidence for better adjustment of 2D-SWE compared to TE for the assessment of liver stiffness and indirectly liver fibrosis [9].
The aim of the study was to analyse the consistency between 2D-SWE stiffness and fibrosis in liver biopsy in patients with chronic HBV and HCV infections. he secondary aim of the study was to analyse the consistency between liver stiffness in 2D-SWE and TE measurements in patients with chronic hepatitis B and C.
Material and methods
Studied population
The study compares the results of hepatic stiffness assessment with 2D-SWE to available past liver biopsy reports and FibroScan results in 153 patients with chronic HBV (n = 51) and HCV (n = 102) infection. The period between the prior biopsy and SWE examination ranged from 0 to 156 months; in 48 patients it was shorter than 24 months, while in 62 patients it exceeded 24 months. In 43 patients with both hepatitides HBV (n = 8) and HCV (n = 35) we performed FibroScan on the same day as 2D-SWE; 8 of these patients also had available a past liver biopsy report. In 16 HCV-infected patients with clinically symptomatic liver cirrhosis we did not perform liver biopsy, but only 2D-SWE. These patients were not generally included in the analysis. However, to eliminate potential bias of focusing on less severe fibrosis, we performed additional AUROC analysis including these patients.
Clinical parameters
The following parameters were included in the analysis: age, gender, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), total bilirubin, leukocyte count, erythrocyte count, haemoglobin, platelet count and prothrombin time.
Calculation of APRI and FIB-4 index
The APRI score was calculated as previously descri-bed: (AST/upper limit of normal)/PLT (109/l) × 100 [10]. The FIB-4 index was calculated as formerly reported: [age (years) × AST (IU/l)]/[PLT (×109/l) × √ALT (IU/l)] [11].
2D-SWE using supersonic shear-wave elastography
2D-SWE was performed using the Aixplorer ultrasound system (Supersonic Imagine, France). Liver stiffness was measured in kilopascals (kPa) and expressed in a 0-4 scoring system, corresponding to the manufacturer-recommended histologic METAVIR scale related to the stage of liver fibrosis, with appropriate ranges separately proposed for HBV, HCV infections and non-alcoholic steatohepatitis (NASH). It is worth noting that the proposed cut-offs have been changing as the results of larger studies providing new data became available. Currently (since 2015), manufacturer-recommended cut-offs for HCV infection and NASH indicate F0-1 for < 7.1 kPa, F2 for 7.1-9.2 kPa, F3 for 9.2-13.5 kPa, and F4 for liver stiffness of > 13.5 kPa. In HBV infection the proposed cut-offs are: < 7.1 kPa for F0-1, 7.1-8.1 kPa for F2, 8.1-11.5 kPa for F3 and > 11.5 kPa for F4 [9].
Transient elastography
One-dimensional TE using a FibroScan device was performed in 43 patients from this group on the same day as 2D-SWE. The measurements were performed according to the recommendation of the manufacturer with an M or XL probe. The results of properly carried out procedures, at least 10 measurements with interquartile range (IQR)/median < 0.3, were taken for the analysis. Liver stiffness was measured in kPa and expressed in a 0-4 scoring system, corresponding to the manufacturer-recommended scale related to the stage of liver fibrosis, with appropriate ranges separately proposed for HBV and HCV infections. Recommended cut-off values for HCV infections indicated F0-F1 for < 7.1 kPa, F2 for 7.1-9.5 kPa, F3 for 9.5-12.5 kPa, and F4 for liver stiffness of > 12.5 kPa [12]. In HBV infection the proposed cut-offs are: < 7.2 kPa for F0-1, 7.2-9.4 kPa for F2, 9.4-12.2 kPa for F3 and > 12.2 kPa for F4 [13].
Liver biopsies
Histologic examinations including fibrosis evaluation of specimens obtained with liver biopsies were analysed by an experienced histopathologist and expressed in the Scheuer classification, which was converted to METAVIR. Results were recognized as consistent with each other if the difference between biopsy and SWE scores did not exceed ±1.
Results
Patient characteristics
General patient characteristics for the HBV group (n = 51) and HCV group (n = 102) are presented in Table 1. Among patients with both HBV and HCV infection majority were male (60.8% and 62.7% respectively) with a mean (±SD) age of 39.7 ±12.1 for the HBV-infected group and 48.5 ±12.6 for the group infected with HCV. In biopsy the most frequently occurring activity grades in both groups were A1 (HBV = 52.6%, HCV = 40.3%) and A2 (HBV = 23.7%, HCV = 47.2%), and similarly the most common stage of liver fibrosis was F1 (HBV = 78.9%, HCV = 61.1%). The mean levels of ALT, AST and GGT were significantly lower (p < 0.001) in patients infected with HBV (38.6 ±42.1, 36.4 ±37.1 and 27.3 ±28.4 respectively) than HCV (73.4 ±75.4, 60.6 ±38.7 and 79.9 ±78.4 respectively). APRI score and FIB-4 index were significantly higher in patients infected with HCV (p < 0.001 and p = 0.004 respectively), whereas the AAR score in this group was significantly lower (p = 0.029).
Table 1
Patient characteristics in HBV and HCV groups
[i] AAR – aspartate aminotransferase and alanine aminotransferase ratio, ALP – alkaline phosphatase, ALT – alanine aminotransferase, APRI – aspartate aminotransferase to platelet ratio index, AST – aspartate aminotransferase, FIB-4 – fibrosis index based on 4 factors, GGT – γ-glutamyl transpeptidase, PT – prothrombin time
Liver stiffness 2D-SWE measurements
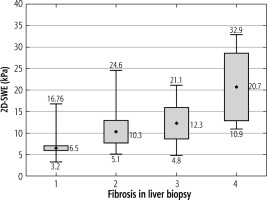
Liver stiffness varied from 3.4 to 62.5 (10.1 ±8.7 kPa) and in the enrolled population was significantly lower (p < 0.001) in patients infected with HBV (8.8 ±9.5 kPa) than HCV (10.8 ±8.3 kPa) (Fig. 1).
Stiffness values analysed in the whole studied population demonstrated a significant positive correlation with the stage of liver fibrosis in biopsy (r = 0.555, p < 0.001). It was particularly significant in HCV infection (r = 0.602, p < 0.001), but not in HBV (r = 0.360, p = 0.026) (Table 2).
Coherence of 2D-SWE measurements and fibrosis in liver biopsies
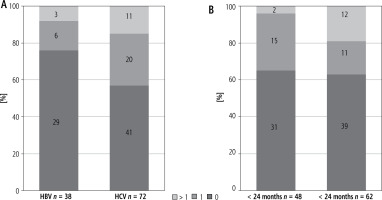
Consistency was 96% (46/48), if 2D-SWE was carried out within 24 months since liver biopsy. In this group 65% (n = 31) of cases achieved exactly the same result of fibrosis in 2D-SWE and liver biopsy, while in 31% (n = 15) the score differed by 1 and 4% (n = 2), which were recognized as non-consistent due to the score difference exceeding 1. The only two inconsistent patients were HCV infected, so consistency in the HCV group was 93% (28/30), whereas in HBV infection reached 100%. Consistency of 81% (50/62) was found if the period between procedures exceeded 24 months (Δ > 24 months). In 19% (n = 12) of cases the difference was more than 1 degree, in 18% (n = 11) it was 1, and in 63% (n = 39) the results were the same (Fig. 2).
Diagnostic performance of 2D-SWE
We additionally assessed the diagnostic accuracy of 2D-SWE in our studied group with predictive models. ROC curves and AUROC were estimated for diagnosing minimal fibrosis (F1), moderate fibrosis (F2), severe fibrosis (F3) and liver cirrhosis (F4) (Fig. 1). Diagnostic performances according to the AUROC values were classified traditionally as excellent (AUROC 0.9-1.0), good (0.8-0.9), fair (0.7-0.8), poor (0.6-0.7) and fail (0.5-0.6). The diagnostic performances of 2D-SWE according to AUROC values were good for discriminating minimal and moderate fibrosis to excellent for the diagnosis of severe fibrosis and liver cirrhosis, especially in our population of HCV-infected patients (Table 3).
Table 3
AUROC values for diagnosis of liver fibrosis
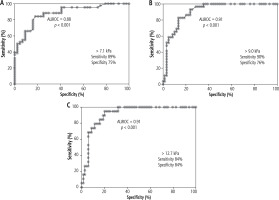
We estimated the ROC curves for HCV patients to assess the sensitivity and specificity of manufacturer-proposed cut-off values discriminating stages of fibrosis in our population (Fig. 3). The mean time between procedures in HCV infection was 1174 days (range: 0-4751 days) and in 30/72 patients the time between procedures was shorter than 24 months.
Fig. 3
ROC curves and diagnostic performance by AUROC estimated for HCV patients (including patients with clinically confirmed liver cirrhosis) discriminating stages of liver fibrosis: A) ≤ F1 vs. ≥ F2, B) ≤ F2 vs. ≥ F3, C) ≤ F3 vs. ≥ F4
We were not able to properly assess the diagnostic accuracy of 2D-SWE in HBV infection, mainly due to the sample size in each fibrosis group being inadequate for comparison. Moreover, the long time between liver biopsy and 2D-SWE in HBV patients (mean: 890 days, range 0-3440 days), exceeding 24 months in 20/38 patients, seemed to strongly bias the results.
Coherence of 2D-SWE and TE measurements
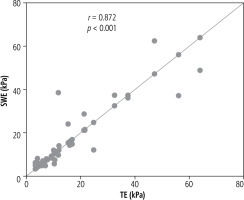
In 43 patients with properly performed 2D-SWE and TE the coherence between these two methods was high (r = 0.872, p < 0.001) (Fig. 4). Consistency of the results of fibrosis in 2D-SWE and TE was 95% (41/43). In 2 cases (5%) the difference was more than 1 degree, in 3 cases (7%) the difference was 1 and in 38 cases (88%) the results were the same.
Other factors influencing the results of 2D-SWE
Spearman’s correlation showed a significant positive correlation of liver stiffness values with AST (r = 0.472, p < 0.001), ALP (r = 0.529, p < 0.001) and GGT (r = 0.570, p < 0.001). Furthermore, stiffness values showed moderate correlations with APRI score (r = 0.558, p < 0.001) and FIB-4 index (r = 0.532, p < 0.001). On the other hand, PLT (r = –0.457, p < 0.001) and PT (r = –0.475, p < 0.001) negatively correlated with mean SWE values.
Discussion
Developing non-invasive methods for the assessment of liver fibrosis which could replace, at least in part, invasive histopathological techniques has been a challenge for many decades. In the field of hepatology, there have been partially successful attempts to provide serological markers that reflect the extent of liver fibrosis. The last years have brought a breakthrough in this field. Rapidly evolving, ultrasound-based techniques showing high accordance with liver biopsies, that allow one to repeatedly assess and follow the progression of liver fibrosis, have been introduced to the market.
So far, the accuracy of 2D-SWE has been validated for patients with chronic HBV or HCV infection and NASH. However, as the results from individual studies were collected, new data influenced the suggested cut-off levels for different stages of fibrosis. In our study performed on 110 patients with chronic HCV or HBV infection with liver biopsy-proven progression of fibrosis and 16 HCV-infected patients with clinically confirmed liver cirrhosis, 2D-SWE showed good to excellent diagnostic performance and high coherence with liver biopsy, especially in HCV infection (AUROC: 0.83-0.97). Generally, coherence was slightly higher in HBV compared to HCV-infection (92% vs. 85%). Moreover, when the liver biopsy and 2D-SWE were performed within 24 months, the accuracy was 93% in chronic hepatitis C and 100% in chronic hepatitis B. However, in HBV infection we were only able to estimate AUROC values for less advanced fibrosis. The diagnostic performance of 2D-SWE was fair in discriminating between fibrosis stage ≤ F1 and F2 ≥ (AUROC = 0.763). We were not able to estimate AUROC values in more advanced fibrosis, mainly due to the long time between liver biopsy and 2D-SWE and inadequate sample sizes. Our results from a single site are comparable to a large multicentre meta-analysis from several countries in Europe and from China [9]. In this latter study Herrmann et al. observed the high concordance of 2D-SWE measurement and stage of fibrosis in liver biopsies taken within a 24-week period [9]. In this study the diagnostic performance of 2D-SWE was good in discriminating between fibrosis stage ≤ F1 and F2 ≥ (AUROC = 0.863) and excellent for the discrimination of more advanced fibrosis in HCV infection (AUROC > 0.9). In chronic HBV infection the diagnostic performance of 2D-SWE assessed in a group of 400 patients, allowing the consecutive stages of fibrosis to be determined, was excellent (AUROC > 0.9).
In our study we observed a strong significant correlation of 2D-SWE and liver biopsy results (r = 0.627, p < 0.001) and a very strong significant correlation between 2D-SWE and TE (r = 0.872, p < 0.001), confirming the high coherence of these two non-invasive methods. The study of Paul et al. on chronic viral hepatitis B and C showed a moderate significant correlation between 2D-SWE and TE [14]. On the other hand, the analysis of Herrmann et al. underlined the superiority of 2D-SWE’s diagnostic performance over TE in the assessment of liver fibrosis [9]. The meta-analysis by Fu et al. demonstrated that the pooled sensitivity and specificity of SWE (88% and 91% respectively) for staging early fibrosis (F1) are similar to those of TE (83% and 89% respectively). However, the overall sensitivity and specificity for detecting significant fibrosis (F ≥ 2) was higher for SWE than other elastography techniques, such as TE [15].
The cut-off values proposed by Hermann et al. in patients infected with HCV are 7.1 kPa for significant fibrosis, 9.2 kPa for severe fibrosis and 13.0 kPa for cirrhosis, with a sensitivity level of 94.7%, 90.3%, 85.8%, respectively, and a specificity level of 52.0%, 76.8%, 87.8%, respectively. In HBV patients those values are 7.1 kPa, 8.1 kPa and 11.5 kPa, respectively, with a sensitivity range of 79.9% to 94.9% and specificity ranging from 73.1% to 93.3% [9]. The cut-off point for significant fibrosis proposed by Dhyani et al. is slightly higher, with the value of 7.29 kPa, sensitivity of 95% and specificity of 51% [16]. In our study, in HCV patients we established values similar to those proposed by Hermann et al., with a cut-off value of 7.1 kPa for F2, 9.0 kPa for F3 and 12.7 kPa for F4, and with sensitivities of 84-90% and spe-cificities of 75-84%. However, in patients infected with HBV we were only able to estimate a cut-off value of 5.6 kPa for F2 (sensitivity 87.5%, specificity 50.0%). We were unable to estimate cut-off values in more advanced fibrosis due to the small sample size and diversity.
Other clinical variables, such as AST, ALP, GGT, platelet count and prothrombin time, may influence the results of 2D-SWE. Likewise, there was a strong positive correlation of APRI score with ALT (r = 0.829, p < 0.001) and GGT (r = 0.703, p < 0.001), as well as a moderate significant correlation with activity grade and fibrosis stage in liver biopsy (r = 0.525 and r = 0.488 respectively). FIB-4 index correlated strongly with GGT (r = 0.659, p < 0.001) and moderately with activity and fibrosis in liver biopsy (r = 0.547, p < 0.001 and r = 0.552, p < 0.001 respectively). Furthermore, there was very strong positive correlation between APRI score and FIB-4 index (r = 0.877, p < 0.001). Both APRI score and FIB-4 index correlated moderately with liver stiffness in 2D-SWE (r = 0.558, p < 0.001 and r = 0.532, p < 0.001 respectively).
It is extremely important, from the clinical point of view, to screen repeatedly a large population such as HBV- or HCV-infected patients. Liver biopsies do not offer such an opportunity. Therefore, the new non-invasive comprehensive techniques for the assessment of liver tissue stiffness are indispensable for the clinical management of patients.
Conclusions
Liver biopsy is the gold standard diagnostic modality for liver fibrosis. However, nowadays, as the availability of non-invasive techniques is growing, this procedure is less suitable to be routinely used and repeated in general clinical practice due to procedure-associated morbidities, such as bleeding, perforation, and infection.
In our study liver stiffness measured with 2D-SWE showed good consistency with the stage of liver fibrosis in liver biopsies, particularly in HCV-infected patients and when the period between procedures did not exceed 24 months.
2D-SWE offers repeatable, quantitative, real-time, two-dimensional elastography by incorporating an advanced ultrafast imaging technique that has a high correlation with histologic staging of liver fibrosis.