Introduction
Psoriasis is a common skin disease, which is considered an immune-mediated systemic disorder [1]. Moreover, the role of genetic and environmental aspects is also highlighted in the pathogenesis of psoriasis [2]. Several studies have clearly documented that not only skin lesions but also pruritus have a huge negative impact on the patients’ quality of life [3]. Pruritus is observed in approximately 70% to 90% of individuals suffering from psoriasis, and some of them complained of a generalized itching [4]. In spite of the high frequency of this symptom, the cause of itching sensations in psoriasis patients still remains unknown.
It is believed that pruritus may be provoked by local inflammation, imbalance in neuropeptides, overexpression of some interleukins, vascular abnormalities or the opioid system dysfunction [4].
Interleukin 31 (IL-31) is a cytokine expressed in various human tissues and is involved primarily in Th2-type inflammatory response [5]. It is produced not only by Th2 cells but also Th1 cells, keratinocytes, mast cells, dendritic cells, eosinophils and fibroblasts [6, 7]. This novel interleukin acts through a receptor complex composed of oncostatin M receptor β and IL-31 receptor α [5]. The stimulation of IL-31 receptor induces secretion of many cytokines and chemokines and therefore it contributes to the recruitment of polymorphonuclear cells, T cells and monocytes to the site of skin inflammation in vivo [6, 8]. Several studies emphasized a possible role of IL-31 in the pathogenesis of itch [5, 8, 9]. It possibly stimulates itching by activation of the IL-31 receptor on sensory nerve cells and promotes nerve fibre elongation [10]. The overexpression of this cytokine in transgenic mice induced pruritic skin condition, which was similar to human atopic dermatitis [6]. According to the available data, IL-31 is strongly associated with chronic pruritic skin diseases (atopic eczema, prurigo nodularis, chronic urticaria), and it may represent a potential target for therapy [5, 9].
In the literature, there are only a few studies concerning IL-31 in psoriasis [8, 11, 12]. Moreover, the role of IL-31 and its gene polymorphisms in the disease pathogenesis and induction of pruritus in psoriasis patients still remains elusive.
Aim
The aim of this research was to investigate the genotype and allele frequencies of IL-31 promoter gene polymorphisms (-1066 G/A and -2057 G/A) and serum IL-31 concentration in relation to the disease severity and pruritus intensity in psoriasis patients from northern Poland and compare the results with the control group.
Material and methods
Participants
This pilot study included 300 adult (161 men and 139 women; mean age: 43.9 years, range: 18–74 years), unrelated patients suffering from chronic plaque psoriasis who were admitted to the Department of Dermatology as well as to the Dermatology Outpatient Clinic of the Medical University of Gdansk. The recruited patients had not been treated systemically for psoriasis (methotrexate, retinoids, cyclosporine, photochemotherapy, corticosteroids) at least for the previous 3 months and had not received topical medications for the previous 1 week. Patients treated with biologics, suffering from other dermatoses, systemic inflammatory disorders or malignancies were excluded.
Recruitment for the control group included 186 adult (77 men and 109 women; mean age: 33.0 years, range: 18–61 years), healthy (mainly blood donors), unrelated volunteers, without psoriasis and other systemic chronic inflammatory cutaneous diseases and allergic disorders as well as without any positive family history of psoriasis. The detailed clinical characteristics of the participants are shown in Table 1. All participants were exclusively of Polish descent (the population of northern Poland).
Table 1
Clinical characteristics of patients with psoriasis and the control group
The Independent Bioethics Commission for Research of the Medical University of Gdansk approved the research (NKBBN/313/2017). The study was conducted in accordance with the Helsinki Declaration and each subject signed written informed consent before participating in the research.
Assessment of psoriasis severity and pruritus
The psoriasis diagnosis was based on detailed dermatological examination. The disease severity assessment was performed using the Psoriasis Area and Severity Index (PASI). According to PASI, patients were divided into mild (PASI < 10 points), moderate (PASI 10–15 points) and severe (PASI > 15 points) psoriasis groups. Diagnosis of psoriatic arthritis was confirmed by a rheumatologist in 30 (10%) patients. Intensity of itch was measured with the Visual Analogue Scale (VAS) as an average pruritus from the previous week (scale from 0 to 10 points). Patients were also divided into two subgroups: early-onset psoriasis (the psoriasis onset age < 40 years; n = 228) and late-onset psoriasis (the psoriasis onset age > 40 years; n = 72).
Identification of IL-31 -1066G/A (rs11608363) and -2057G/A (rs6489188) gene polymorphisms
Genomic DNA was prepared from whole-blood samples using Blood DNA Prep Plus in accordance with the protocol of the manufacturer (A&A Biotechnology, Gdynia, Poland). The IL-31 gene polymorphisms were analysed by amplification refractory mutation system PCR (ARMS-PCR) method, using self-designed specific sequences of oligonucleotides. As the internal amplification control, the growth hormone 1 (GH1) gene was applied. PCR conditions were as follows: initial denaturation for 5 min at 94°C; 34 cycles of 40 s at 94°C, annealing step for 60 s at 64°C for IL-31 -2057, 62°C for IL-31 -1066 and 90 s at 72°C; final elongation at 72°C for 5 min. PCR products were separated in 2% agarose gel.
Evaluation of serum IL-31 levels
Serum levels of IL-31 were assessed using an enzyme-linked immunoabsorbent assay (ELISA) standard kit (BioVendor-Laboratorni medicina a.s., Brno, Czech Republic). This product has been tested by Quality Control and passed internal specifications. All procedures were conducted based on the manufacturer’s instructions. Serum IL-31 levels were analysed in randomly chosen 80 adult patients from the psoriasis group: 44 (55%) males and 36 (45%) females; and 70 healthy subjects from the control group: 39 (55.7%) males and 31 (44.3%) females.
Statistical analysis
Statistical analysis was performed using Statistica 10.0 software package (StatSoft, Inc., 2011). The χ2 analysis was used to compare the observed number of genotypes with that expected for a population in a Hardy-Weinberg equilibrium as well as to examine the significance of the differences in the observed genotypes and alleles between study groups. A logistic regression model was applied to calculate the odds ratio (OR) and the 95% confidence interval (CI). Differences in median values between the groups were analysed with the Mann-Whitney U test and the Kruskal-Wallis test. The correlation coefficients were evaluated using the Spearman’s rank correlation test. P-value < 0.05 was considered to be statistically significant.
Results
Pruritus
Pruritus was observed in 97.4% of patients with psoriasis and its average intensity assessed using VAS was 6.2 ±2.1 points. The intensity of itch sensations in early- and late-onset psoriasis was similar (6.1 vs. 6.4 points, respectively), whereas in psoriasis patients with psoriatic arthritis, the mean VAS was 7.1 ±1.4 points.
IL-31 promoter gene polymorphisms in relation to the clinical course of the disease, pruritus and serum IL-31 concentration
The genotype and allele frequencies of IL-31 gene polymorphisms in patients and control subjects are shown in Table 2.
Table 2
The occurrence of genotypes and alleles for IL-31 -1066G/A and -2057G/A gene polymorphisms in psoriasis patients and the healthy control group
The -1066 AA genotype of the IL-31 gene was statistically more often observed in patients and it increased the risk of psoriasis (OR = 1.80; p = 0.04). There were no significant differences in frequency of other genotypes and alleles for -1066G/A IL-31 in psoriasis patients and control subjects.
The GG genotype as well as G allele in the IL-31 -2057 polymorphism were significantly rarer in psoriasis and were associated with a decreased risk of the disease (OR = 0.6, p = 0.007 and OR = 0.7, p = 0.01, respectively).
There were no differences in frequencies of genotypes and alleles for both studied polymorphic variants in psoriasis patients in relation to the disease onset, presence of psoriatic arthritis and family history of psoriasis (Table 3) as well as no correlation with pruritus intensity, clinical severity of the disease or serum IL-31 level (Table 4).
Table 3
Type of psoriasis, presence of psoriatic arthritis and family history of the disease in relation to genotypes and alleles for IL-31 -1066G/A and -2057G/A polymorphisms
Table 4
Pruritus intensity, disease severity and serum IL-31 levels in relation to genotypes for IL-31 -1066G/A and -2057G/A polymorphisms
Serum IL-31 levels in correlation with the clinical course of the disease and pruritus
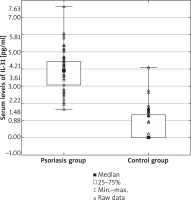
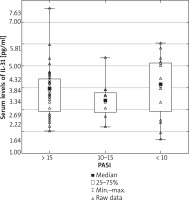
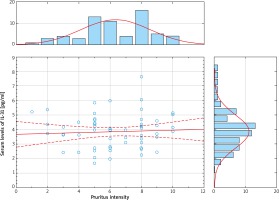
Serum levels of IL-31 were significantly elevated in psoriasis patients, compared with healthy controls (psoriasis group: median 3.95; mean: 3.88 ±1.08 pg/ml; range: 1.64–7.63 pg/ml vs. control group: median: 0.00, mean: 0.59 ±0.88 pg/ml; range: 0.00–4.10 pg/ml; p < 0.000001) (Figure 1). Nevertheless, serum IL-31 concentration did not correlate with the severity of psoriasis (Figure 2), pruritus intensity (Figure 3) as well as disease onset and presence of psoriatic arthritis.
Discussion
Although pruritus is a relatively common symptom of psoriasis, its aetiology is not clearly understood. According to Czarnecka-Operacz et al. [12], 88.3% of patients with psoriasis vulgaris complained of pruritus and the most frequent factor exacerbating itching sensations was stress (39.6%). Moreover, the authors documented that pruritus in the course of psoriasis was independent of sex, disease duration and PASI. In that study the average itch intensity in psoriasis measured using VAS was 4.9 ±2.9 points, corresponding to moderate pruritus [12]. Similarly in our study, almost all (97.4%) psoriasis patients complained of itch and its average severity was assessed as moderate (6.2 ±2.1 points). In other research, 81% of pruritic psoriatic subjects reported itching limited only to psoriatic lesions [13]. Nakamura et al. [14] observed that psoriatic lesional skin from patients with itch is more innervated in the epidermis and in the superficial dermis compared to non-pruritic psoriatic skin.
Interleukin-31 is considered to be expressed by activated Th2 cells and significantly associated with induction of pruritus in patients with atopic dermatitis [15–17]. It was documented that expression of IL-31 and IL-31 receptor A (IL-31RA) is increased within affected skin as well as in peripheral blood of patients with atopic dermatitis [18]. Subcutaneous injection of nemolizumab (anti-IL-31RA antibody) remarkably inhibits pruritus in atopic dermatitis patients without serious side effects [19]. Increased serum IL-31 levels were also noted in primary cutaneous T-cell lymphomas (CTCL) and it was even suggested that pruritus in CTCL may be related to IL-31 [20]. Moreover, this interleukin was overexpressed in the affected skin of lichen planus, but its expression did not correlate with intensity of itching sensation [21].
The significance of IL-31 in pruritus in psoriasis has not been determined yet. Nattkemper et al. [11] documented that IL-31 had an increased gene transcript level in itchy psoriatic skin. There are only two studies evaluating the concentration of IL-31 in serum of psoriasis patients. The research performed by Narbutt et al. [8] demonstrated an increased serum level of IL-31 in psoriasis (p < 0.0001), which decreased upon UVB irradiation. However, no correlation between serum IL-31 level and itch intensity was observed [8]. On the other hand, Czarnecka-Operacz et al. [12] did not reveal any difference in the cytokine serum concentration between patients and controls (p = 0.359), but confirmed no significant relationship between serum IL-31 and PASI or pruritus intensity. Due to the conflicting data between IL-31 levels in psoriasis vs. controls in both studies as well as a low number of participants enrolled, we decided to substantiate these observations by increasing the study group. Our findings confirmed a meaningful increase of IL-31 in serum among patients and therefore proved a possible role of this cytokine in the pathogenesis of psoriasis but not revealed a significance of IL-31 as a biomarker of psoriatic pruritus. Lack of direct correlation between serum IL-31 level and pruritus intensity may be explained by the fact that IL-31 is not a single pruritogenic and sensory nerve growth factor acting within skin. As indicated, this cytokine activates a distinct transcriptional program in sensory neurons, leading to nerve branching and their elongation in vitro as well as in vivo [22]. In the context of psoriasis pathogenesis, IL-31 can be involved in the development of psoriatic lesions due to its possible influence on angiogenesis and chronic inflammation. As reported, IL-31 has been considered a proangiogenic factor inducing the expression of vascular endothelial growth factor (VEGF) in epithelial cells [23, 24]. The effect of IL-31 on release of many proinflammatory cytokines and their role in the recruitment of inflammatory cells to the site of inflammation should also be highlighted [5].
The polymorphic variants of the IL-31 gene have been analysed in some cutaneous disorders [25–29]. Lan et al. [26] showed an association between specific alleles of IL-31 and atopic dermatitis. Sokołowska-Wojdyło et al. [27] reported that the AA genotype and allele A for the IL-31 -1066 as well as GA genotype for the IL-31 -2057 are associated with an increased risk of atopic dermatitis development. In addition, a significant correlation between genotype GA and GG of IL-31 -2057G/A polymorphism and moderate pruritus was also confirmed. Interestingly, another study demonstrated the association between the severity of atopic dermatitis, intensity of pruritus or serum IL-31 levels in a Polish population and some specific haplotypes of the IL-31 gene [28]. The two distinct IL-31 gene polymorphisms (-1066 and -2057) were also assessed in mastocytosis. In adult patients AA genotype for the IL-31 -2057 was associated with a higher risk of occurrence of mastocytosis [29]. However, there were no statistically significant differences in serum IL-31 levels as well as pruritus intensity in respect of the -1066G/A and -2057G/A gene polymorphisms in adult patients with mastocytosis and healthy subjects [29]. A recent genome-wide association study identified IL-31 (locus 12q24.31) as one of the remarkable candidate genes located within newly indicated psoriasis susceptibility loci, what can serve as a worthwhile direction of future studies into psoriasis pathogenesis [30]. Our research is the first one concerning the significance of IL-31 -1066G/A and -2057G/A promoter gene polymorphisms in psoriasis patients. The results showed that the presence of the -1066 AA genotype of the IL-31 gene was statistically more frequent in patients and it increased the risk of psoriasis. The -1066G/A and -2057G/A polymorphisms are localized in the promoter region of the IL-31 gene, and therefore they may affect gene expression and upregulate the cytokine level. Presumably, because many genetic (epistasis) and epigenetic factors may influence the final protein level, our study did not reveal a significant correlation between presence of analysed polymorphisms and IL-31 level in serum. Moreover, we present a pilot study, so the sample size might constitute the limitation of our findings. The paper of Schulz et al. [25] revealed that the degree of IL-31 mRNA expression was significantly higher (3.8-fold) in activated peripheral blood mononuclear cells from healthy carriers of three genotypes: IL-31-2057GG,-1066AA and IVS2+12AA as compared with non-carriers. In our study, we compared the serum IL-31 level between patients carrying both IL-31-2057GG and -1066AA genotype with non-carriers, however, we have not found statistical differences between compared groups. The IL31-1066 is located within a consensus site for GATA binding protein 3 (GATA-3) and is predicted to obliterate the binding site of this transcription factor [25]. As indicated, GATA-3 is involved in the differentiation of Th2 cells as well as keratinocytes and is significantly downregulated within psoriatic skin [31]. Therefore, triggers affecting altered GATA3 expression and function may play an important role in the pathogenesis of psoriasis.
Studies assessing the correlation between many genetic markers and psoriasis have provided different results for various populations [32]. Moreover, the heterogeneity may be observed even between different cohorts of European origin. This may be a consequence of population-specific effects, different genetic backgrounds or varying environmental exposures. Therefore, Riveira-Munoz et al. [32] highlighted the value of examining genetic risk factors for psoriasis in multiple populations. We present the first association study of the IL-31 gene in psoriatic patients from Poland. To our knowledge, this is also the first report demonstrating the association of the IL-31 gene with psoriasis in patients from Eastern Europe.
Conclusions
Our research is the first one that assessed the significance of IL-31 -1066G/A and -2057G/A promoter gene polymorphisms in psoriasis pathogenesis. In the light of the contradictory literature data on IL-31 serum levels in psoriasis, our findings confirmed a meaningful increase of IL-31 in serum among patients. Based on our data as well previous studies it seems that the serum concentration of IL-31 may not be a reliable marker of psoriatic pruritus. However, further investigations on the interaction of IL-31 and the skin barrier in susceptibility to psoriasis are strongly required.