Introduction
Malignant neoplasms are one of the most frequently reported side effects in post-transplantation patients. The risk in adults is significantly increased – 10 times more than in the general population [1, 2]. According to ANZDATA (Australia and New Zealand Dialysis and Transplant Registry), the standardized incidence ratio (SIR) was calculated at 3.5. SIR is the incidence of a certain type of neoplasm, which would occur per 100,000 people in a given population, provided that its age structure was equal to the one of a standard population [3]. Data published in 2011 by the US Organ Procurement and Transplantation Network (OPTN), which had been gathered from 175,000 patients who underwent kidney (58%), liver (22%) heart (10%) or lungs (4%) transplantations, have shown that neoplasms were diagnosed in 10,656 of them (6%) and that SIR for all neoplasms was calculated at 2.1. There have been 30 most common types of neoplasms, however, a significant increase was attributed to several of them: Kaposi’s sarcoma (SIR 61), skin cancers (SIR 13.9), non-Hodgkin lymphomas (SIR 7.5), liver neoplasms (SIR 11.6), vulvar neoplasms (SIR 7.6) and lip neoplasms (SIR 16.8) [4].
Recent publications provide little information about neoplasia incidence in organ recipients in paediatric population. The majority of researchers who studied the topic have observed that the risk of developing a malignant neoplasia is increased by three times among children after kidney, liver, heart or lungs transplantation [5].
In children, the most frequently diagnosed type of cancer is the post-transplant lymphoproliferative disease (PTLD), with skin cancer being the second. Furthermore, there is reportedly an increased incidence of thyroid papillary cancer, ovarian tumours and Kaposi’s sarcoma [6].Skin cancer is relatively infrequent in paediatric patients, nonetheless, the risk of the aforementioned increases significantly 10 or more years after the transplantation, which is when the child reaches adulthood. It is not uncommon for a patient, who has undergone a transplantation in childhood, to require another transplantation as an adult. In such cases, skin cancers usually become a problem approximately 25 years after receiving the first transplant, however some malignancies can potentially occur much sooner [7].
The post-transplantation carcinogenesis in children patients, as well as in adults, is caused by a combination of different risk factors, including among others: their originalimmunocompetence, type, dose and duration of immunosuppressant therapy and a potential infection by oncoviruses. It seems, however, that the chronic post-transplantation immunosuppression would have the biggest impact. It is indicated by the clinical observation showing that neoplasms occur more frequently after heart or lung transplantation in comparison to kidney or liver transplantation, which is in direct connection to stronger immunosuppression administered in the former [7].
Methods
A recently-conducted, large-scale literature search aimed to find data on skin cancer in paediatric organ recipients. Search terms included “paediatric solid organ transplantation”, “skin neoplasms”, “child”, “transplant recipients”, “melanoma”, “ paediatric transplant”, “adolescent”. PubMed was the main search engine. Additional articles were identified through reference search of the articles acquired via PubMed.
Risk factors
The post-transplantation carcinogenesis in children patients, as well as in adults, is caused by a combination of different risk factors, including among others: their original immunocompetence, type, dose and duration of immunosuppressant therapy and a potential infection by oncoviruses. It seems, however, that the chronic post-transplantation immunosuppression would have the biggest impact. It is indicated by the clinical observation showing that neoplasms occur more frequently after heart or lung transplantation in comparison to kidney or liver transplantation, which is in direct connection to stronger immunosuppression administered in the former [7]. Other risk factors involve: Fitzpatrick skin type I to III, exposure to UV (ultraviolet) radiation, history of non-melanoma skin cancer, history of leukaemia or lymphoma before or after transplantation, biological treatment and voriconazole used.
Population of children after transplantation
For several years, there has been an increase in the number of children who underwent organ transplantations, most of all kidney and liver transplantations. It has become more frequent to perform lung, heart or heart-and-lung transplantation in paediatric patients [8]. Organ recipients below 18 years of age account for 4–7% of all organ recipients. The average age of children receiving organ transplants is 11 [9]. The average age of children undergoing liver transplantation is lower (2.5 years) than of those receiving kidney transplantation (12.7 years). Regarding heart transplantations, there are two major age groups: infants, who present heart failure due to congenital conditions and teenagers who have been diagnosed with progressing cardiomyopathy [10]. In the US, children after liver transplantations account for 12.5% of all post-liver transplantation patients [11]. In paediatric patients, there has been observed a lower male-to-female ratio when compared to the adult population 1.2 : 1 [12, 13].
Immunosuppressant therapy in paediatric population
Like in adults, the majority of children, who underwent kidney and heart transplantation, are treated according to the treatment regimen which contains 3 immunosuppressant drugs and is generally stronger than the one administered after liver transplantation. However, it is also worth mentioning that the multi-centre research, which aimed to compare the effectiveness of immunosuppression based on two (prednisolone (P) and tacrolimus (TAC)) and three drugs (prednisolone, cyclosporine A (CsA) and azathioprine (AZA)), has shown that there were fewer cases of transplant rejection in the group of patients who had been treated with tacrolimus [14]. In most cases, the same immunosuppression regimen is maintained until puberty. In the population of adolescent children it is vital to be wary of the impact that immunosuppression has on children’s growth, their fertility and safety of their potential pregnancy [15].
In recent years there has been a change in frequency of using certain types of immunosuppression. This tendency is observed both in adult and paediatric patients. Agents with immunosuppressive and potential anti-proliferative properties, mTOR inhibitors, are more commonly utilized [16]. The newest immunosuppressive drugs, mTOR kinase inhibitors (e.g. rapamycin (sirolimus) and everolimus (EVR)), are introduced to patients’ post-transplantation therapy to a greater extent. They are especially recommended for children who have a greater risk for malignancies or have already been diagnosed with one. These drugs have shown great anti-proliferative and anti-oncogenic potential and effectively reduce the risk of post-transplantation neoplasia [17].
Recent investigations have shown that the results of a 3-year course of treatment consisting of everolimus and small doses of cyclosporine A (CsA) and prednisolone (P) are indeed very promising, considering its very good tolerance by patients and its great effectiveness. There has not been a single case of rejection of the transplanted organ [18].
Another research confirms undoubtedly that treatment consisting of a 3–6 ng/ml dose of everolimus and a reduced dose of cyclosporine A may be as effective as the standard therapy based on calcineurin inhibitors (CNIs) combined with mycophenolate mofetil (MMF). It was observed that there was a smaller risk of rejection of the transplanted organ as well as improvement in function of the transplanted kidney. Furthermore, reducing the dose of CsA thanks to introducing everolimus is an excellent solution for children after kidney transplantation, increasing the effectiveness and safety of immunosuppressant therapy [19].
This therapy may potentially facilitate a withdrawal of steroids from treatment. It also reduces the risk of cytomegalovirus CMV infection and prevents polyomavirus (BKV) replication. However, arterial hypertension and dyslipidemia, which are common side effects of everolimus, may account for an increase in mortality in children treated with this agent [19].
The effect of immunosuppressant drugs on carcinogenesis in children is relatively little known [17]. The majority of patients after organ transplantation, in which cancer occurred, have received treatment based on prednisone (92%) azathioprine (72%) and cyclosporine A(53%), a regimen widely used many years ago [11].
Introduction of stronger immunosuppressants (tacrolimus), including more common use of monoclonal antibodies (anti-thymocyte globulin), has provoked an increase in malignancies prevalence, especially in post-transplant lymphoproliferative disease (PTLD). It is claimed that the duration of immunosuppressant therapy, including the one used in immunosupressive treatment of primary disease (e.g. glomerulonephritis), as well as the general strength of immunosuppression (which is a combined effect of all drugs used in a certain protocol), are primary causes of carcinogenesis [19].
Adolescent patients’ appearance is another very important aspect. The Cushingoid appearance or obesity, which are common adverse effects of corticosteroids, are hardly accepted in this specific population. In addition, other conditions like: hirsutism, gingival hypertrophy or sebaceous glands hypertrophy, which are side effects of CsA, may significantly worsen their self-acceptance. MMF is a reason of relatively few cosmetic adverse effects and seldom deteriorates the quality of young patients’ life [14].
Skin cancer in paediatric organ recipients
Medical literature data indicate that skin cancer is the most common malignancy occurring in paediatric patients who underwent kidney transplantation and the second most common tumour after non-kidney transplantations [11]. The most frequently observed are squamous cell carcinoma (SCC) and basal cell carcinoma (BCC). They usually occur 12–15 years after organ transplantation, on average at the age of 26–28.
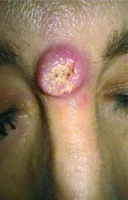
SCC may present in two different clinical forms: as an exophytic tumour or a penetrating ulceration. Unlike in the general population, in patients after organ transplantation, SCC is usually characterized by a rapid growth and presence of multifocal lesions. They are frequently observed as flat, elevated, erythematous lesions with superficial peeling, which are commonly mistaken for chronic inflammation [20–23] (Figure 1).
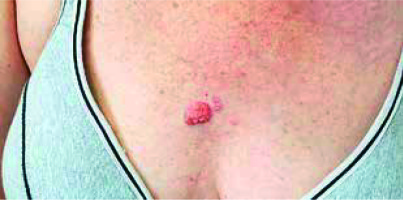
BCC is a slowly growing, locally malignant epithelial skin cancer. It may present in the following forms: nodular, pigmented, ulcerative, sclerosing, cystic and superficial [23]. The course of BCC in patients after organ transplantation resembles the one in general population. The most common type of BCC is the nodular type, which usually presents as a pearl-shaped papule or a nodule with visible telangiectasias, surrounded by a pearly ridge. It occurs mostly in sun-exposed areas such as head and neck [24] (Figure 2).
There has been only a few scientific publications of a greater significance, which assessed the incidence of skin cancer in children after organ transplantation. The results are summarised in Table 1.
Table 1
Skin cancers observed in paediatric solid-organ transplantation population
The largest from the above mentioned is the research of Penn et al., who described 135 cases of skin cancer in children after transplantation: 54 SCC, 19 BCC, 16 SCC + BCC, 12 cases of melanoma, 19 cases of anogenital area cancer, and 15 cases of Kaposi’s sarcoma. Gruber et al. have observed 5 cases of skin cancer: 4 SCC and one of anogenital area [25]. Coutinho et al. have presented12 cases of skin cancer in children after a kidney transplantation: 7 SCC, 3 BCC, and 2 melanomas. The research of Bernstein et al. contains only one case of SCC post-heart transplantation. Ozen et al. presented one case of Kaposi’s sarcoma in a child after a renal transplantation [26, 27]. The last research was published by Euvrard et al., who described four cases of skin cancer in children after organ transplantation: 3 BCC and 1 BCC + SCC. Euvrard et al. described a group of 225 patients; 76% of them were kidney transplant recipients in childhood. None of them developed skin cancer in childhood, however four of them were diagnosed with skin cancer in early adulthood, on average at the age of 28. In cancer patients there were four cases of BCC and also one of SCC [16]. In the remaining publications, the authors reported only few cases of skin cancer in children after transplantation. All of them were single-case reports. One of them was a 15-year-old boy who developed squamous cell skin cancer (SCC) of the lower lip, 2.5 years after a heart transplantation. He received the transplant due to cardiomyopathy caused by the toxic effect of doxorubicin, which was prescribed to him a few years before in the course of Burkitt’s lymphoma [11, 26]. The youngest patient, who was diagnosed with skin cancer suffered from Fanconi anaemia. This congenital immunodeficiency predisposed the patient to develop a malignancy since early childhood [28]. In one of Koukourgianni’s publications, a group of patients who underwent a kidney transplantation in their childhood was observed. In a patient with haemolytic-uremic syndrome, 10 years after a kidney transplantation, a SCC and BCC of the eyelid area was found. A 17-year-old patient with thrombosis presented with BCC of the scalp 3 years after receiving the transplant. Another case was a child after kidney transplantation due to steroid-resistant nephrotic syndrome. He developed a premalignant condition – Bowen’s disease 11 years after the transplantation, located on the back of the hand. In all three cases surgical removal of affected skin areas was applied [6]. In another study of Simard et al. there were two cases of NMSC, 1 case of melanoma and 3 anogenital area tumours identified within the group of 536 patients under 18 years old [29].
Melanoma is much less common in children than in adults after organ transplantation (12% vs. 5%). This diagnosis may occur much sooner than other types of skin cancer. Fourteen cases of melanoma have been reported so far in persons who underwent an organ transplantation in childhood [2, 11]. Half of these cases were diagnosed in childhood. In the above mentioned study, 25% of patients died due to that illness [11]. It is believed that a total number of melanocytic naevi in patients after organ transplantation increases, which may be a risk factor for melanoma. It is worth mentioning that in general population, 25% of cases of melanoma arise from naevi and the remaining 75% occur de novo in previously unchanged skin. In post-transplantation patients, however, this ratio increases to as much as 37% of melanoma cases that arose from naevi [24].
An increase in the number of naevi occurring after an organ transplantation is very often due to immunosuppressive treatment [30, 31]. Similar cases are observed in patients with immunodeficiency e.g. due to AIDS [32]. A significant increase in the number and size of naevi was observed in 7.5% of children after organ transplantation. However, no dysplastic naevi were observed [33]. Additionally, treatment with recombinant growth factor, which is supposed to improve the growth process in children after transplantation and those affected by chronic kidney disease, may also increase the number of naevi [12, 34].
It seems that a chronic immunosuppression may alter the dermatoscopic image of melanocytic naevi, increasing its atypical features [12, 34]. It is confirmed by the case of a 16-year-old boy, who received immunosuppression (prednisone, azathioprine, cyclosporine A) after kidney transplantation, and presented a rapid, generalized, disseminated outbreak of numerous melanocytic naevi (ca. 500) with aggregated melanocytes in dermatoscopic examination. Multiple brown granules were observed in most of the naevi. Microscopic examination revealed a low-grade dysplasia. There was no history of a long-term sunlight exposure. There was no family history of either melanocytic naevi or melanoma. During four years of clinical and dermatoscopic observation of the naevi, there were no signs of progression to melanoma. After several years, a kidney graft rejection occurred and the patient required dialysis again. Immunosuppression was immediately discontinued and a gradual regression of naevi was observed within the following months [35].
Kaposi’s sarcoma is also more common in children who are organ recipients than in general paediatric population. In Penn et al. study, which assessed post-transplantation children population, 15 out of 135 patients with malignancies were diagnosed with Kaposi’s sarcoma. In Ozen et al. study there was only one case of Kaposi’s sarcoma after renal transplantation. Kaposi’s sarcoma is classified as a malignant neoplasm of mesenchymal origin. Its features are abnormal angiogenesis, hyperplasia of epithelium and endothelium of blood vessels, which become disfigured and inflamed. Clinically it usually affects the skin, however internal organs may also be affected during the course of the disease. There are four clinical forms of Kaposi’s sarcoma: classic, endemic, epidemic and iatrogenic, linked to using immunosuppressant drugs after organ transplantation. In the iatrogenic type, mucocutaneous symptoms are observed. They are usually lesions characterized as plaques or nodules, merged together, which are of dark-blue or purple colour and are predominantly located in the calf area. Mucosal lesions, which are usually seen as maculopapular and purple, most commonly affect the palate. Gingival hypertrophy may also be observed [24]. Aside from skin lesions, the disease also manifests with abdominal symptoms. It may appear from about 5 months to 17 years after the transplantation but it is most commonly observed several months after the organ transplantation. Similarly to adults, there is a proven link between an infection with HHV 8 and later development of Kaposi’s sarcoma [36, 37].
Anogenital area tumours account for 4% of all tumours occurring in children after organ transplantation. This group consists of labial, scrotal, penile cancer and anogenital area skin cancers. They appear usually after 12 years from the transplantation, on average at the age of 27. They are mostly predominant in women (women-to-men ratio of 8 : 1). In children they usually present as multifocal lesions, unlike in older people. There is a strong connection between Kaposi’s sarcoma and the presence of genital warts [16]. In this study, Penn observed 19 anogenital area tumours in children after kidney transplantation, Gruber et al. 1 case and Simard et al. 3 cases [11, 25, 29].
Prognosis in children with neoplasms after organ transplantation
The most common cause of death in paediatric population after organ transplantation is cardiovascular complications, while in adults it is cancer. The majority of children who were diagnosed with a malignancy after transplantation, die from complications that follow anti-cancer treatment such as encephalopathy, respiratory failure or metabolic disorders [6]. In Koukourgianni’s observation, 25% of patients (4 out of 16) died because of an oncologic disease. First of those patients died because of encephalopathy and respiratory failure in the course of B-cell lymphoma 11 months after kidney transplantation. The second recipient died due to an air embolism that occurred during haemodialysis, in the course of PTLD, 2.5 years after kidney transplant. The third patient died because of a recurrent, severe hypercalcaemia emerging during general anaesthesia in the course of Burkitt’s lymphoma, 6.5 years after kidney transplantation. The last one died because of metabolic disorders due to PTLD, years after the kidney transplantation [6].
Summary
Chronic immunosuppressive therapy in organ recipients among children predisposes to different types of cancer, including skin cancer. The key risk factors for skin cancer are: age at the operation, length of therapy, type and cumulative dose of immunosuppressive treatment used, infection with oncoviruses and exposure to UV radiation. The standard procedure in most skin cancers is surgical removal of the tumour with an appropriate margin of healthy skin. Also, recommendations include reduction in the dose of the immunosuppressive drugs and introduction of the new-generation antiproliferative drugs – mTOR inhibitors (e.g. rapamycin and everolimus).
However, the most important factor is the prevention based on limiting the sunlight exposure from the early childhood on. It should also be advised to use UV-filters and to wear appropriate, sun-protective clothing. Regular, repeated education of children and parents is of the utmost importance. Apart from the prophylaxis, an early cancer screening should be applied in the population of organ recipients. It is possible owing to a close cooperation with other specialists, among others a dermatologist.