Despite generalized pustular psoriasis (GPP) is a rare and potentially life-threatening variant of psoriasis.
Generalized pustular psoriasis in childhood has been regarded as less recalcitrant with a more benign course, higher rates of spontaneous resolution and long-term remission compared to adult-onset cases, it still can be a life-threatening condition in paediatric patients [1]. The exact prevalence is unknown and ranges from 0.6% to 7% of paediatric psoriasis cases, with a male predominance and onset between 3 and 16 years of age [2]; these discrepancies are probably due to the historical lack of consistency in differentiating between forms of pustular disease, including GPP and localized conditions, such as palmoplantar pustulosis and acrodermatitis continua of Hallopeau [1, 3]. GPP is characterized by extensive, macroscopically visible sterile pustules with overlying erythematous plaques with or without systemic inflammation, including fever, chills and leucocytosis [1, 4]. The pathophysiology of pustular psoriasis has not been fully elucidated up to now; it has been associated with abnormalities in the cytokine interleukin-36 receptor-antagonist signalling [3, 5]. Most cases of pustular psoriasis are idiopathic, although a number of potential triggers have been identified, primarily drug-related ones or triggers such as viral or bacterial infections, autoimmune diseases and neoplasia [2, 5].
Herein, we present the case of a 3-year-old male presented with severe pustular psoriasis that began shortly after Infanrix hexa (diphtheria (D), tetanus (T), pertussis (acellular, component) (PA), hepatitis B (rDNA) (HBV), poliomyelitis (inactivated) (IPV) and Haemophilus influenzae type b (Hib) conjugate vaccine) vaccination in January 2020. Biopsy from the representative lesion was consistent with the diagnosis of pustular psoriasis. Due to further progression of symptoms, despite the use of topical glucocorticoids and systemic antibiotics, cyclosporine was administered for 6 months. The therapy was discontinued due to severe flare-up during treatment. Methotrexate was implemented in order to control the disease symptoms which led to a partial response for almost 18 months. In January 2022, after a gastrointestinal infection, there was an exacerbation of the disease that was not responding to standard rescue therapy (Figure 1). The patient’s initial physical examination upon referral to us revealed an acutely ill-appearing child. On examination there were generalized, thick, scaly erythematous plaques, studded with pustules covering up to 80% of the body surface area (BSA). The patient experienced severe cutaneous pain. Laboratory tests showed raised total white cell count of 23.9 × 109/l (4.5–13 × 109/l) with neutrophilia of 18.2 × 109/l (2.5–8.9 × 109/l) and an elevated C-reactive protein of 96.1 mg/l (0–5 mg/l). The patient was assessed as Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) scoring 4 (severe) and Generalized Pustular Psoriasis Area and Severity Index (GPPASI) scoring 53.4. We administered anti-tumor necrosis factor-α (anti-TNF-α) monoclonal antibody (adalimumab) 20 mg every 2 weeks combined with acitretin 10 mg daily. We saw a rapid improvement in general condition without significant side effects (Figure 2). Three weeks after beginning adalimumab therapy, there was nearly total resolution of his psoriatic lesions. No pustules and very few plaques were seen. Overall health had improved and the cutaneous pain experienced by the patient had diminished. Taking into account the good clinical response, the combination of adalimumab and acitretin has been maintained without exacerbations so far. The patient did not experience any further flare-ups. He remains clear 12 months following the initial presentation.
Figure 1
Exacerbation in the course of generalized pustular psoriasis after lack of response to methotrexate: widely distributed eruption of pinhead-sized pustules arising on inflamed, erythematous skin
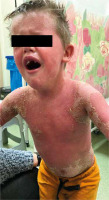
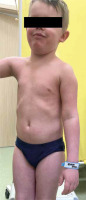
Figure 2
Combination therapy of adalimumab and acitretin was associated with clinical improvement of skin lesions
GPP is a rare form of psoriasis. Because of the acute onset of symptoms and the potentially fatal outcome of pustular psoriasis, a therapy that is highly effective, easy to administer and safe for the patient and with a rapid onset of action should be selected. There is a paucity of randomized controlled trials and standardized guidelines for children; most of the recommendations are based on the case reports and/or case series [1]. In general, first-line therapies for childhood GPP include acitretin, acitretin in combination with oral prednisone, methotrexate, and cyclosporine, while drugs such as adalimumab and infliximab are considered the second-line therapy for juvenile GPP. Likely it is due to relative lack of data on safety in paediatric population. Recently, the anti-TNF-α, adalimumab has shown efficacy in adult patients with pustular psoriasis; however, there is lack of evidence of its usage in the paediatric population. Another burdensome challenge is the lack of a standardized method of monitoring disease response to therapy and the lack of high-quality data on treatment efficacy. Biologics may be an attractive option as they offer convenient dosing schedules with limited laboratory monitoring, and have targeted effects that may reduce potential end organ toxicity [1]. Biologics have been used successfully in the treatment of refractory juvenile GPP. Etanercept has been considered a possible first-line therapy despite the lack of direct comparisons between adalimumab so far [6, 7].
Data from the literature, based predominantly on single case reports and case series, highlight high efficacy and rapid onset of action of adalimumab in childhood onset GPP [2, 5, 7–10]. Due to GPP common resistance to conventional systemic therapy as more experience is gained, anti-TNF-α agents may become first-line agents for juvenile GPP. We present the use of anti-TNF-α monoclonal antibody in combination with acitretin for the effective treatment of paediatric GPP. In our case, the clinical symptoms disappeared rapidly and remission remains without any clinically significant exacerbations for 12 months so far, which is a better result than we achieved during cyclosporine treatment. In line with other reports, adalimumab in combination with a low dose of acitretin is effective and tolerable in the treatment of refractory GPP. Subcutaneous injection of adalimumab every other week in the treatment of children with GPP has significant clinical efficacy with rapid clearance of skin lesions, providing a novel alternative for children with pustular psoriasis who responded poorly to traditional treatment or are not suitable for traditional treatment. Controlled studies are warranted to corroborate our observation.








