Introduction
Hidradenitis suppurativa (HS) (acne inversa) is a chronic disease characterized by recurrent, painful, deep-seated nodules and abscesses of apocrine gland-bearing skin [1]. HS causes painful discomfort as well as social embarrassment, which has a negative influence on the quality of life [2].
A mean disease incidence of 6.0 per 100,000 person-years and an average prevalence of 1% have been reported in Europe [3]. Presentation of the disease involves a classic distribution of painful lesions in the skin creases with associated local complications of abscesses and sinuses [4]. There are often systemic symptoms, and management is handled by various specialties such as dermatology, general surgery, and plastic surgery, rather than a single specialty. The multidiscipline disease is often diagnosed with delay. The exact etiology remains unknown, but it is suggested to be associated with smoking, diabetes, poor hygiene, immunocompromised and reduced cutaneous levels of calprotectin, zinc, or ascorbate [5, 6]. There is also involvement of genetic mutations in the γ-secretase genes NCSTN, PSENEN and PSEN1. The aforementioned genes are also connected with psoriasis vulgaris [7, 8]. HS is associated with several comorbidities, including diabetes mellitus and Crohn disease. Clinical presentation of hidradenitis suppurativa ranges from rare, mild inflammatory nodules to widespread abscesses, sinus tracts, and scarring. The primary mechanism is a follicular occlusion with secondary inflammation, and destruction of the pilo-sebaceo-apocrine apparatus with extension to the adjacent subcutaneous tissue. It results in an inflammatory response that is associated with bacterial infection of the apocrine sweat glands [9]. Consequently, the abscesses extend deeper into the subcutaneous tissue and then intercommunicating sinus tracts develop, resulting in irregular scars [10].
The most common sites include axillary, inguinoperineal, gluteal, submammary regions and the nape of the neck, waistband, and inner thighs [11]. Medical treatment with topical or systemic antibiotics is widely used as a first-line defense in the management of mild disease in early stages. In severe cases surgical treatment should be considered. In early stages of abscess formation simple incision and drainage of abscesses and deroofing of sinus tracts are frequently performed [12]. Skin grafting and closure with cutaneous or myocutaneous flaps are often performed in plastic surgery departments. The disease is not well known by surgeons, which occasionally may result in poor medical decisions. The paper presents the most common surgical techniques in the treatment of severe HS.
Surgical methods
There is no perfect method in surgical treatment. Surgical treatment is considered the best choice for advanced hidradenitis suppurativa [13, 14]. It is also reported that early wide surgical excision is important and effective in order to prevent complications and the recurrence of hidradenitis suppurativa and to improve the quality of life [14].
Currently, there is still no consensus regarding the optimal surgical strategy. A recent systematic review and meta-analysis reported that there is insufficient evidence to show the advantages of flaps over skin grafts although they are superior to primary closure [15].
Unfortunately, surgical treatment is often performed years after the initial symptoms of the disease, after numerous ineffective cycles of pharmacotherapy [16].
One should always consider time of healing, possible complications, and patient outcomes. We would like to present incisions, skin grafting techniques and local flap reconstructions – the most common methods used in the Centre for Burn Treatment in Siemianowice Śląskie.
Incision and drainage techniques
The purpose of incision and drainage is pain relief in case of a tense, fluctuating acute abscess. There is only a short-term effect, because lesions treated by incision and drainage tend to relapse. It is the easiest and best known technique in surgery departments [17].
Deroofing
The deroofing technique was first described by Mullins and colleagues in 1959 [18]. In the 1980s, the technique was modified by preservation of the exposed lesion floor. Deroofing is the primary surgical therapy for persisting nodules or sinus tracts in HS Hurley stage I/II.
The procedure starts with identification of the HS lesions to be deroofed by physical examination. Wounds are then left for secondary intention healing and spontaneous epithelialization.
The standard deroofing procedure involves removal of the roofs of sinus tracts and scraping off gelatinous and sanguinolent material, but does not remove adjacent fibrous and scar tissue. Deroofing has mostly been used as a tissue-sparing technique for mild to moderate forms of HS. It is a safe method that does not require special equipment (electrocoagulation, basic surgical tools) and is easy to conduct. It can be performed in dermatosurgical departments [17].
Modified deroofing followed by meticulous sinus tract removal is a surgical approach suitable for patients with moderate disease, especially in the axillary region. This results in a low recurrence rate and the same healing period as that of the standard deroofing procedure [19].
Skin grafts
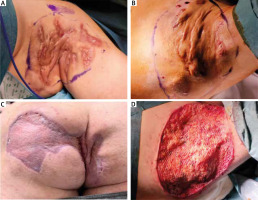
After surgical excision of hidradenitis suppurativa, reconstruction with a skin graft or a flap is performed when primary closure is not possible. Split-thickness skin grafts, usually meshed, are used typically in large wounds [20, 21]. In surgery departments, skin grafts are usually harvested by the dermatome (mainly donor thighs), and then they are expended in ratios of 1 : 1.5 to 1 : 2 or even 1 : 3 in the case of major deficiency of the tissue. Skin grafts are a good choice to close the wide wound after excision. Considering that hair follicles and sweat glands are involved in the etiology of the condition, split-thickness skin grafting (STSG), which does not contain cutaneous appendages, might be a good wound closure technique. Disadvantages of the method are donor site wound and contracture scars where the skin grafts are placed (especially in the axilla and groin). In our opinion, skin grafts are a fair choice in the buttocks. The method can be performed in plastic/general surgery departments. Wide excision of the HS lesions and successful reconstructions with skin grafts are presented in Figure 1.
Local flaps
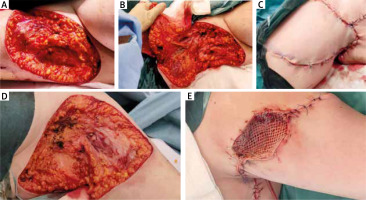
Local flaps are a good option to close the wound after excision. Radical excision of all affected skin followed by flap coverage of the defect is the treatment method of choice in severe and recurrent cases. However, the use of flaps to prevent less favorable functional results was introduced at an early stage. A review of the Limberg flap for axillary hidradenitis was presented quite recently [22]. Local fasciocutaneous V-Y advancement flaps were reported for large defects following wide surgical excision of long-standing hidradenitis suppurativa of the axilla [23]. Some flaps may be indicated in particular cases such as the use of a thoracodorsal artery perforator flap (TDAP) in axillary HS [24, 25]. Local flaps are tensionless technique. Covering HS defects with conventional flaps is not without disadvantages, however. First, a tendency toward more limited excision results in high recurrence rates in the short term due to inadequate excision of the pilosebaceous glands. Scars are wider, due to wide preparation of the flaps. Nevertheless, this tensionless technique might be considered in the axilla and groin. Moreover, large local flaps raised to cover large HS defects are associated with perfusion problems because of folding of the base of the flap or compression of the flap at the axilla or groin. Excision, preparation of the local flap and successful reconstruction are presented in Figure 2.
Discussion
The literature on surgical treatment of hidradenitis suppurativa is huge and the review of this disease goes back to the 1950s [17]. Surgery is often necessary in severe and recurrent cases of HS when medical treatment fails. The most important determinant of success is complete resection of the lesion [26]. Patients with Hurley stage III or severe stage II may benefit more from surgical than medical treatments [27]. Complete wide resection of all affected hair-bearing skin, subcutaneous fat, and deep fascia ensures that all infected apocrine glands, subcutaneous abscesses, sinuses, and tracts are removed [28]. Therefore, less invasive techniques such as local incision and drainage of the abscesses or limited excision and primary closure of the lesions due to inability to perform flap surgery are likely to fail [29, 30].
Complete resection of HS leaves a large defect that may not be amenable to primary closure. A variety of techniques, such as secondary healing, skin grafting techniques, and local flaps, have been used to close the defects [31–37]. A recent meta-analysis showed a 27% recurrence rate after the deroofing procedure, while the largest open study yet conducted (by Van der Zee et al.) showed a recurrence rate of 17% [38]. According to a recent systematic review and meta-analysis by Mehdizadeh et al., postoperative recurrence rates for hidradenitis suppurativa were 15% for primary closure, 8% for skin flaps, and 6.0% for skin grafting [15]. Watson noted the need for reoperation in approximately 20% of patients with inguinal disease whether they were treated with excision and primary closure or excision and skin grafting [39]. Moosa et al. reported a 37% recurrence rate for the inguinal area [40]. Excision and split skin grafting is a basic tool in the surgical treatment and the result of this procedure is often satisfactory [41–43]. Skin grafts after excision of HS provide simple closure of large defects. However, the results are disfiguring due to color variability, depression, and contractures resulting from the removal of all subcutaneous tissue. Graft failure is another common problem in areas that are prone to shearing forces, such as the axilla, perineum, groin, and buttocks [44, 45]. Hynes et al. and Chen et al. mentioned that covering HS defects with conventional flaps is not without disadvantages, however. First, a tendency toward more limited excision results in high recurrence rates in the short term due to inadequate excision of the pilosebaceous glands [32, 33].
Soldin et al. stated that excision of all hair-bearing skin and flap coverage is the method of choice for treatment of HS [31]. In their recent study, Varkarakis et al. reported eight problems in a series of 15 patients reconstructed with Limberg flaps following wide resection of HS. Recently local, regional, and free flaps, such as the lateral thoracic flap, the fasciocutaneous V-Y flap, the Limberg flap, and the musculocutaneous flap, have been used for this purpose. In particular, perforator flaps, such as the thoracodorsal artery perforator (TDAP) flap, have been reported as advantageous for the reconstruction of the soft-tissue defect following excision of severe extensive axillary HS [46]. Kagan et al. performed a special algorithm of surgical interventions in HS patients [47]. The algorithm is based on area of involvement, and extensive or localized disease [39]. There have been few case reports in the literature regarding surgical treatments of HS lesions in the submammary area. Moosa et al. reported the most aggressive treatment of HS by performing bilateral mastectomies [40]. There is no doubt that this approach of treatment is mainly dependent on the size and the site of the defect. Fertitta et al. performed a study on satisfaction with HS surgical treatment. In all, 42 (75%) patients were completely satisfied in recommending surgery to another patient (75%); 61% were completely satisfied with accepting another operation in the same area and 66% for another location; and 69 (81%) were completely satisfied that the surgery was the best treatment for them [48]. Fertitta’s study is very optimistic, but more studies are necessary in this field.
Despite the method of reconstruction, the hospitalization period can be reduced, thus reducing the cost of treatment. It is of great importance to determine the timing of wide surgical excision and the selected method of reconstruction. During the acute phase, surgical drainage and irrigation with the administration of antibiotics should be the mainstay of treatment [13].
Conclusions
Surgical treatment of HS, regardless of disease severity, is considered as a good choice for curative intervention. Collaboration between dermatologists and surgeons is necessary. HS is a debilitating chronic skin inflammatory disease which can greatly impact quality of life. HS is often diagnosed late. The study shows that surgical treatment should be considered in HS (Hurley stage II, III). We described the reconstruction of HS defects at various sites using local flaps, skin grafts and wide excision with secondary granulation healing. There is no perfect technique for covering these large defects after excision. Surgical treatment in HS should be carried out by surgeons who have thorough knowledge of the pathology of the disease and excellent surgical skills. In our opinion local flaps are a good option for coverage of large excision wounds. Nevertheless, more scientific studies must be prepared in this area, especially with long follow-up and an exact recurrence rate in each technique. Our opinion is that primary closure with flaps or skin grafts should be the method of choice. Extensive severe HS is treated with excision of the affected tissue and the surgical defect is left to heal by secondary intention (it is beginning to be a historical method). Experience showed that secondary wound healing with granulation is not satisfactory, mainly for the patients, due to the long time to heal. We recommend local flaps as the best surgical choice in this burdensome disease.