Low molecular weight heparins (LMWHs), particularly enoxaparin, are administered routinely to Coronavirus disease 2019 (COVID-19) patients, in order to treat or prevent the associated, possibly serious coagulation disorders [1].
Both immunoglobulin E (IgE)-mediated and non IgE-mediated systemic reactions to LMWHs are considered rare [2–4]. Particularly, a single case of the drug-related reaction with eosinophilia and systemic symptoms (DRESS) has been documented in the literature [4].
Here we report on an elderly COVID-19 patient who developed DRESS upon prophylactic treatment with enoxaparin. Etiological diagnosis was corroborated by the lymphocyte proliferation test (LPT) [5] upon recovery. The management strategy adopted is outlined and commented.
A 95-year-old female resident of a nursing home developed acute respiratory symptoms, at the peak of the COVID-19 “first wave” in Italy in March 2020. Upon hospitalization, infection by SARS-COV2 was confirmed by nasopharyngeal swab, followed by polymerase chain reaction (PCR) detection of the Coronavirus. Chest computed tomography revealed diffuse bilateral infiltrates. At admission, blood counts were in the normal range (including eosinophils, 70/μl). The elevated erythrocyte sedimentation rate (87 mm/h) and C-reactive protein (18.7 mg/l) were the only abnormal laboratory values. Underlying comorbidities included type 2 diabetes and hypertension (both moderate and well controlled). Upon hospitalization, she was treated (empirically) with hydroxychloroquine (400 mg/day) and enoxaparin (4,000 UI/day). Her conditions remained fair for 2 weeks (with no need for oxygen therapy), when she presented with a maculopapular skin eruption with a purpuric aspect and scaling, involving more than 50% of the body surface (abdomen, back, upper and lower limbs), accompanied by severe pruritus and burning sensation. Fever (> 38.5°C), lymph node swelling at multiple peripheral stations and elevated serum creatinine values completed the clinical picture. The withe blood cell count reached 15,160/μl, with eosinophil count at 1,540/μl. A DRESS diagnosis was established (RegiSCAR score = 7) [6]. Thus, hydroxychloroquine, often associated with severe drug hypersensitivity, including DRESS [7, 8], was discontinued. Enoxaparin dosage was doubled (8,000 IU/day), in consideration of increased D-dimer values (2.173 μg/ml). Prednisone (25 mg/day) and cetirizine (10 mg/day) were added to the therapy. However, a further deterioration of the clinical conditions occurred, leading to replacement of enoxaparin with the fully synthetic pentasaccharide factor Xa inhibitor fondaparinux (2.5 mg/day, subcutaneously), generally well tolerated in patients with local non-immediate cutaneous reactions to LMWHs, including enoxaparin [9, 10].
Management of the case according to this therapeutic schedule led to a slow resolution of maculopapular lesions, over approximately 1 month, with a substantial fever decline achieved in 2 weeks and apyrexia in 3 weeks. Finally, the eosinophil counts also declined steadily and normalized by day 30 after the emergence of the DRESS eruption. Prednisone and fondaparinux were then discontinued.
A few days later, the patient was discharged, upon double successive negative nasopharyngeal swab.
Precision diagnosis: Following a 7-day corticosteroid wash-out period, a blood sample was obtained from the patient (fully recovered; at home). Peripheral blood mononuclear cells were isolated by gradient centrifugation (800 x g, 45’) on Lympholyte® (Cedarlane, EuroClone, Milan, Italy), upon plasma removal and suspension of the blood cellular moiety in Dulbecco’s phosphate buffered saline. Mononuclear cells were maintained in Dulbecco’s modified Eagle’s medium, with 10% autologous serum (v/v), with streptomycin (100 μg/ml), at 37°C, in a 5% CO2, vapour-saturated atmosphere, in 64 cm2 glass Petri dishes, for 4 days, in order to allow clearance of the monocyte-macrophage component. Successively, micro-cultures were generated with the resulting purified lymphocytes (6 × 104 cells in 200 μl) and employed for carrying out LPT for enoxaparin and fondaparinux, respectively, under culturing conditions as above. LPT was performed essentially as described [11]. Briefly, triplicate micro-cultures were incubated with the two drugs, respectively, at three different ten-fold concentrations: therapeutic concentration (TC; calculated on the drug distribution volume), TC/10 and TCx10 (defect and excess concentration, respectively). The distribution volume and the TC were 5.24 l and 15 mg/l, for enoxaparin [12], and 9 l and 0.27 mg/l for fondaparinux [13]. Triplicate micro-cultures incubated with phytohemagglutinin-M (from Phaseolus vulgaris; 2.25 μg/ml) and the medium alone served as the positive and the negative control, respectively. Following 4-day incubation with the drugs, lymphocyte proliferation was assessed upon inclusion of the non-radioactive thymidine analogue 5-bromo-2’-deoxyuridine (BrdU; 100 μM), in the micro-cultures, for 2 h. Incorporation of the nucleotide in proliferating cells was evaluated by an anti-BrdU monoclonal antibody (7.5 U/ml; from Roche Diagnostics GmbH, Mannheim, Germany) [14]. The LPT is deemed positive when the proliferation rate of any of the three concentrations tested compared to the negative control (stimulation index) equals or exceeds 2 [5, 11].
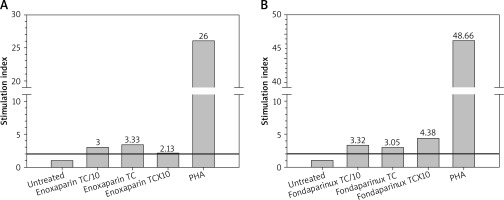
The assay revealed that not only enoxaparin (as suspected), but also fondaparinux induced significant lymphocyte proliferation (Figure 1).
Figure 1
LPT results for enoxaparin (A) and fondaparinux (B). Lymphocyte micro-cultures were exposed to the culprit drugs at therapeutic concentration (TC), TC/10 and TCx10, respectively. The test was consistently positive (stimulation index ≥ 2), for all three concentrations for both drugs. Lymphocyte response to the mitogen phytohemagglutinin-M (PHA) was openly valid
LMWHs, including enoxaparin, have been involved mainly in local delayed hypersensitivity reactions. These reactions are not very infrequent and often mild or moderate. Fondaparinux appears to be tolerated by patients with previous delayed local reactions to LMWHs [8, 10].
Systemic severe delayed reactions, particularly DRESS, are rarer. To our knowledge, this is the second report of a DRESS associated to exposure to enoxaparin and the first one of DRESS associated to fondaparinux. Moreover, by LPT, we showed that the two LMWHs cross-reacted with each other, making fondaparinux an unlikely alternative to enoxaparin in the case of severe delayed systemic reactions (in spite of the fully synthetic structure).
Thus, a full-blown DRESS occurred in an elderly COVID-19 patient (with important comorbidities). This led to discontinuation of the probably useless hydroxychloroquine administration, in fear of a possible causative role. Enoxaparin was then suspected and discontinued. A sustained high dosage corticosteroid treatment was undertaken and the patient eventually recovered from both COVID-19 and DRESS, and survived. Probably correct was the choice of maintaining anti-coagulation by LMWHs. Enoxaparin was indeed replaced with fondaparinux, in the belief that the latter drug had a less allergenic profile. Although, in retrospect, LPT proved it wrong, anticoagulation was probably instrumental in obtaining the healing.








